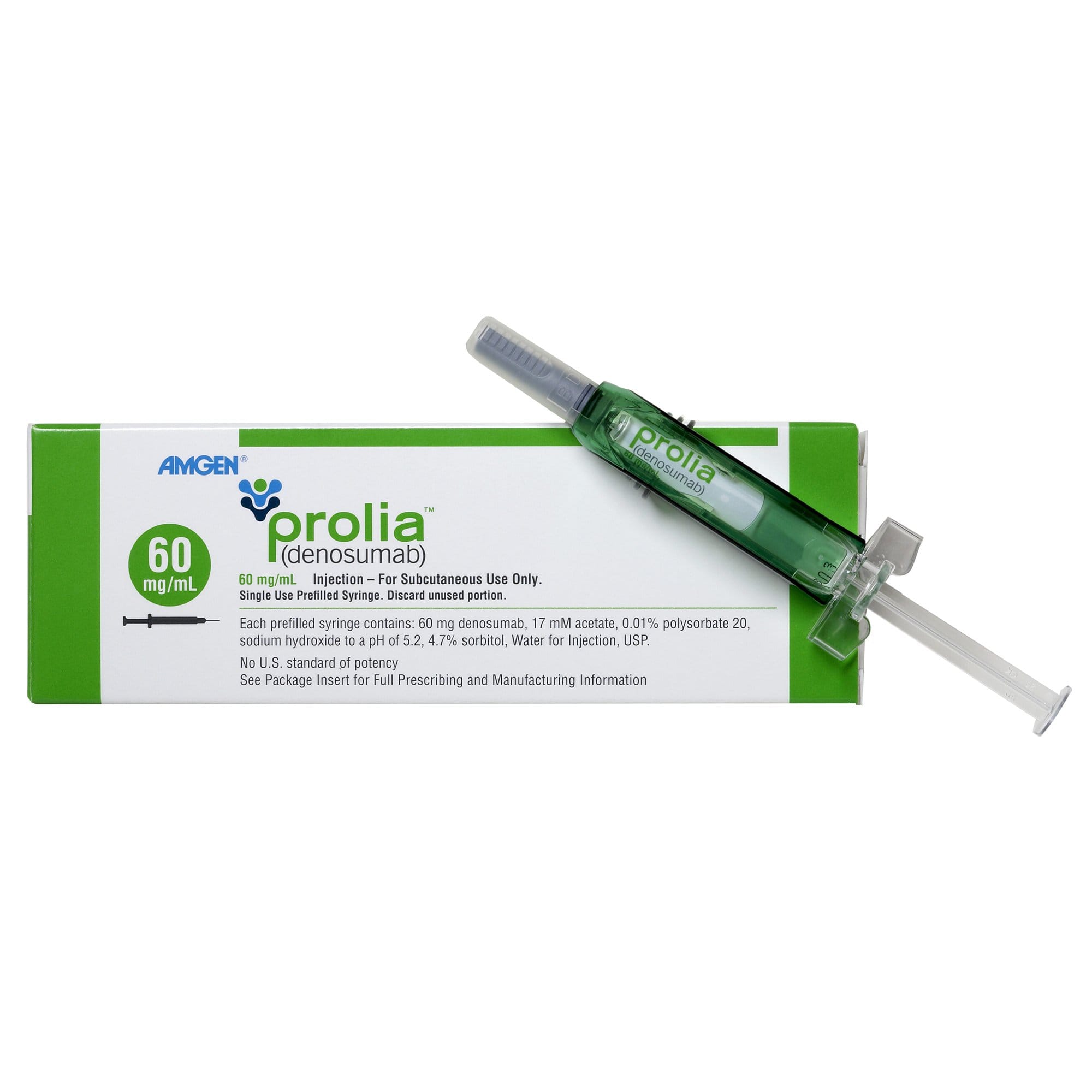
Prolia® Prefilled Syringe for Clinics
$599.00
Description
What Prolia Is and How It Works
Prolia® is a monoclonal antibody supplied as a sterile, single-use prefilled syringe for subcutaneous administration in professional settings. Clinics incorporate this biologic into supervised bone health services overseen by endocrinology and rheumatology teams. We support US distribution for verified healthcare facilities. Med Wholesale Supplies serves licensed clinics and healthcare professionals with authentic, brand-name medical products sourced through vetted distributors.
This agent targets RANKL to influence bone remodeling pathways under clinician oversight. It integrates well into structured injection workflows that include screening, scheduling, and post-administration monitoring. This listing reflects the Prolia English alternative presentation and aligns with documentation preferences common to US-based practices.
Professional Applications
Clinics use this preparation within multidisciplinary osteoporosis and bone health programs. Typical settings include endocrinology, rheumatology, fracture liaison services, and geriatrics where teams coordinate periodic injection visits and follow-up assessments. It can also fit into infusion or biologic clinics that administer subcutaneous products under standardized protocols.
Your team can reference category resources such as Osteoporosis and Rheumatology when planning service lines and inventory. For additional background context, see our in-depth article Bone Health Guide, which summarizes clinical considerations for program design.
Key Features
- Monoclonal antibody class: targeted mechanism supporting bone health protocols.
- Subcutaneous route: suited for clinic-administered injections with observation.
- Sterile, ready-to-use: unit is prepared for immediate professional use.
- Single-use design: reduces cross-contamination risk and simplifies disposal.
- Consistent unit presentation: aids standardization across locations and staff.
- Professional labeling: supports clinical documentation and inventory control.
- Manufacturer-grade quality: produced under established biologics standards.
Benefits in Practice
This product fits clinical operations that value predictability and workflow efficiency. Prefilled presentation streamlines preparation and helps reduce setup time. Consistent unit format supports training, checklist use, and adherence to injection policies. Clear labeling assists receiving, storage, and point-of-care verification. Standardized presentation can improve inventory rotation and simplify reconciliation. The format also supports remote-site deployment with centralized oversight.
Teams can integrate the product into patient calendars, reminder systems, and EMR task lists. Staff may bundle visits with labs or follow-up assessments to increase throughput. The preparation arrives ready to administer, minimizing compounding steps. It supports standardized room turnover and sharps handling. These practical factors can help clinics deliver reliable, repeatable services.
Composition & Ingredients
The active component is denosumab supplied in a sterile aqueous solution for subcutaneous use. This listing represents a clinic-grade biologic, packaged for single use. Exact inactive ingredients are specified on the manufacturer’s labeling and typically include stabilizers and buffers appropriate for injectable formulations. When necessary, consult the latest package insert for full composition details.
For reference and search clarity, this product corresponds to denosumab 60 mg/mL injection as used in professional practice. Review the medication guide and manufacturer labeling for contraindications and precautions relevant to your workflow.
Adverse events seen with this class can include back discomfort, musculoskeletal pain, joint pain, and local injection site reactions. Less commonly reported events include skin or urinary infections and ear infections. Rare but serious risks, as described in authoritative sources, include severe hypersensitivity, osteonecrosis of the jaw, and atypical femoral fractures. Ensure screening, counseling, dental assessments when indicated, and post-injection monitoring follow your clinic’s protocols.
Packaging & Supply
The line is supplied as a single-use 1 mL prefilled syringe designed for immediate use in a clinical setting. Each unit is labeled to support verification and documentation, with lot number and expiry visible on manufacturer labeling to aid inventory management and recall readiness. The device is suitable for subcutaneous injection according to institutional policy and clinician direction.
The presentation delivers a concentration of 60 mg/1 mL per syringe. Do not reuse needles or syringes. Store and handle according to the current manufacturer label and your facility’s procedures. If you need the non-English packaging variant for specific program needs, see our related option Non-English Syringe.
Ordering & Logistics
Account verification is required. Sign in to view eligibility, request a quote, or align deliveries with your receiving hours. We coordinate compliant packing, order consolidation where appropriate, and documentation suitable for institutional records. Support for Prolia US shipping is available to licensed destinations pursuant to applicable requirements.
Many clinics schedule recurring replenishment to match their injection calendar and reduce idle inventory. Your team can also align this biologic with related therapies within Rheumatology service lines. For high-complexity infusion programs, we also carry options like Remicade Non-English that may share similar receiving and storage workflows.
Comparable Products
For clinics evaluating alternatives in the bone health category, consider Evenity Non-English as a class-comparable biologic for program design comparisons. Orthopedic and rheumatology services may also review hyaluronic acid options such as Orthovisc when planning comprehensive joint care offerings. Selection should be guided by prescriber judgment, established pathways, and formulary parameters.
Pricing & Access
Sign in to review available contract tiers for institutional orders. Approved accounts can request quotes, set replenishment preferences, and align invoicing with internal purchasing cycles. If your team benchmarks denosumab price across supply channels, please note that pricing, lead times, and eligibility can vary by account status and allocation. We provide line-item details for procurement review and audit-ready recordkeeping within your purchasing system.
Availability & Substitutions
Supply may vary by lot and allocation. If the requested item is temporarily constrained, we can discuss institution-approved substitutes or staggered fulfillment that matches your injection calendar. Clinics searching to buy denosumab injection can evaluate related listings within Pharmaceuticals or consult our article-level overview Injection Overview. Confirm any substitution with prescribers and your P&T governance before implementation.
Authoritative Sources
- Amgen Prolia HCP – product mechanism, safety, and access resources for professionals.
- FDA Prescribing Information – current US labeling, contraindications, and warnings.
- MedlinePlus: Denosumab – class overview and patient-facing safety information.
For a broader category view, see Osteoporosis resources that may help with formulary planning.
Prolia ships to US for qualified accounts; contact our team to align temperature-controlled handling when required and tracked US delivery with your receiving hours.
Frequently Asked Questions
What handling and storage considerations apply to this biologic?
Follow the current manufacturer labeling and your facility’s biologics policy. Maintain appropriate temperature ranges as specified on the package insert, avoid freezing, and protect from conditions outside labeled limits. Keep the syringe in original packaging until use to preserve labeling and lot traceability. Allow sufficient time for product to reach room temperature per your internal procedures before administration. Do not shake, do not reuse, and dispose of sharps according to institutional and local regulations.
How can our clinic integrate this product into existing workflows?
Use standardized checklists for receiving, storage, verification, and administration. Align order cycles with your clinic’s injection calendar to minimize idle inventory. Train staff on inspection, labeling confirmation, and subcutaneous administration policy. Coordinate visits with labs or assessments to streamline throughput. Document lot numbers and expiry in the EMR or inventory system. Establish contingency plans for delayed shipments or temporary shortages to maintain schedule continuity.
What adverse reactions should staff be prepared to monitor?
Commonly reported reactions include musculoskeletal pain, arthralgia, back discomfort, and local injection site effects. Some users may experience skin infections, urinary infections, or ear infections. Rare but serious risks described by authoritative sources include severe hypersensitivity, osteonecrosis of the jaw, and atypical femoral fractures. Establish clear escalation pathways for any suspected serious reaction and review manufacturer labeling for full safety details prior to administration.
Can this presentation be co-managed with other therapies in our service line?
Yes. Many clinics align biologics across endocrinology or rheumatology services to streamline procurement and handling. Coordinate scheduling, storage capacity, and staffing so injection visits do not compete with infusion chair time. When adding adjunct therapies or vaccines, verify compatibility of visit workflows, observation periods, and storage requirements. Maintain clear prescriber pathways and update your P&T committee on any changes to formulary or protocols.
What documentation supports quality control during receiving and administration?
Record lot number, expiry date, and carton identifiers on receipt and at administration. Use barcode capture where available. Keep the carton and syringe packaging accessible until documentation is complete. Maintain temperature logs per policy and reconcile inventory after each session. Attach relevant education materials to the EMR when appropriate. Retain invoices and delivery records for your purchasing system to support audits and recall readiness.
How should our team plan for demand variability or allocation limits?
Model demand with scheduled injection lists and historic utilization. Maintain a small buffer inventory within your policy limits. If allocation tightens, prioritize scheduled visits, and consider staged deliveries or alternate lots. Communicate early with your distributor, and pre-authorize substitutions if permitted by your P&T governance. Keep patients informed through standardized outreach, and document any rescheduling to maintain continuity of care.
Where can clinicians find authoritative prescribing information and class guidance?
Consult the latest US Prescribing Information for detailed indications, contraindications, warnings, and adverse reactions. Manufacturer HCP portals often provide dosing guides, medication guides, and access resources. Independent references such as FDA databases and MedlinePlus offer class-level insights. Ensure your team works from the most current label and retains links in your EMR or policy repository for quick reference.
Specifications
- Main Ingredient: Denosumab
- Manufacturer: Amgen
- Drug Class: Osteoporosis Treatment
- Generic Name: Denosumab
- Package Contents: 1 x 60 mg/1ml Pre-filled Syringe
- Storage Requirements: Cool Temperature (2℃~8℃)
- Main Usage:
Here to help
Questions about ordering, delivery or products? You can email our team here or call now at 1-800-630-9757 and be connected with your dedicated Account Manager
Related Products
NEAUVIA™ ORGANIC INTENSE LIPS
RejuvaNAD+
Related Articles
Sculptra Side Effects: What Clinics Should Screen and Track
Key Takeaways Tracking Sculptra side effects starts with clear expectations, tight documentation, and consistent follow-up.…
Hyaluronidase for Lip Filler: Clinical Workflow Essentials
Key Takeaways Hyaluronidase for lip filler is typically used to reverse hyaluronic acid gel in…
What Is Mesotherapy? Clinical Uses, Risks, and Workflow
Key Takeaways Define the technique: what is mesotherapy refers to superficial multi-injection delivery of small…
Juvederm Before And After Photos Documentation Guide For Clinics
Key Takeaways Standardize images: Control pose, lighting, lens, and distance. Document context: Record product, lot,…
Esthetician License Requirements for Med Spa Clinic Teams
Key Takeaways Verify licensure status: confirm active status with the state board. Match role to…
Esthetician vs Aesthetician: Clinic Role and Credential Guide
Key Takeaways Spelling varies by region and employer style guides. Licensing rules come from state…
Juvederm For Clinics: Formulations, Safety, and Workflow
Key Takeaways Juvederm is one of the best-known hyaluronic acid (water-binding sugar) filler brands. Clinics…
Esthetician vs Dermatologist Roles for Clinic Teams
Key Takeaways In clinic operations, esthetician vs dermatologist decisions shape safety, delegation, and documentation. Clear…