Interest in Mounjaro weight loss has changed how many clinics plan obesity-care workflows. Tirzepatide is a dual incretin (GIP/GLP-1) medicine, and its use often spans diabetes care and weight management discussions. For practice leaders, the hard part is not the headline results. It is building repeatable processes for patient education, adverse-effect triage, documentation, and product verification.
This briefing focuses on clinic-facing considerations. It also addresses common search-driven misconceptions you may hear in visits, messages, or referrals. Use it to align clinical teams, front desk staff, and procurement on a shared operational approach.
Key Takeaways
- Confirm indication status from current labeling and local policy.
- Set expectations early about variability and tolerance over time.
- Standardize documentation for device type, lot, and patient teaching.
- Use a consistent workflow for verification, receiving, and storage.
- Prepare a structured plan for adverse-effect escalation and follow-up.
Mounjaro weight loss: Clinical Context for Obesity Care
Tirzepatide is prescribed as a once-weekly injectable medicine that targets two hormonal pathways involved in glucose regulation and appetite. In everyday practice, this creates overlap between metabolic disease management and weight-focused care pathways. Your obesity program may see referrals from primary care, endocrinology, and cardiometabolic clinics. That mix makes it important to standardize intake questions, baseline documentation, and follow-up cadence across teams.
Regulatory status matters for how you document goals and coverage. In the United States, tirzepatide has labeling for type 2 diabetes under one brand, and a separate tirzepatide product is labeled for chronic weight management. Policies differ by jurisdiction and payer. If your clinic uses pathway templates, consider building separate note macros for “labeled weight management” versus “off-label weight management,” with clear clinical rationale and shared decision documentation. For broader program planning, it helps to browse your internal formulary alongside a curated Weight Loss Category view to keep options and devices organized.
Why it matters: Documentation gaps can create avoidable delays in follow-up and dispensing.
Trust cue: Access is limited to licensed healthcare entities and professional use settings.
Setting Expectations From Trials to Real-World Practice
Clinicians increasingly field “week-by-week” questions fueled by social posts and influencer timelines. Many patients ask about “before and after 1 month” changes, or share screenshots labeled “weight loss by week.” These materials can be useful for engagement, but they are not a substitute for clinical context. Real-world outcomes vary with comorbidities, concurrent medications, nutrition support, and adherence to follow-up visits.
When patients cite Mounjaro weight loss reviews, consider translating the conversation into measurable, clinic-relevant endpoints. Examples include weight trend consistency, waist measures when appropriate, blood pressure patterns, and lab follow-through. Your staff can also pre-empt confusion by using a one-page handout that explains why early weeks often emphasize tolerability and skills-building (injection technique, meal planning resources, symptom logging). For high-level education materials you may already share, see Weight Loss Injections and how other GLP-1 pathways are commonly discussed in Wegovy And GLP-1 Therapy.
A practical communication tip is to separate “time to see scale movement” from “time to stabilize side effects.” Patients may assume both move together. In reality, appetite changes, gastrointestinal tolerance, and behavior changes can evolve at different rates. Keeping those tracks distinct reduces unnecessary portal messages and reduces dose-chasing behavior.
Adverse Effects and Monitoring Considerations
Frontline teams should expect frequent questions about mounjaro side effects, especially during initiation and after dose escalations. Most discussions center on gastrointestinal symptoms, but clinics should also be ready to review contraindications and label warnings. A structured intake form helps your team capture past pancreatitis, gallbladder history, severe gastrointestinal disease, and relevant endocrine history, without turning every visit into a long interview.
GI symptoms, meal timing, and “how long will this last?”
Patients often report nausea, early satiety, reflux, constipation, or diarrhea. They may also describe mounjaro side effects after eating, especially with large or high-fat meals. Symptom duration varies widely. Many patients describe improvement as they adapt, while others have persistent symptoms that require reassessment. Operationally, your clinic benefits from a consistent symptom log template, including onset timing, hydration status, trigger foods, and any vomiting frequency. That structure lets clinicians answer “how long does mounjaro side effects last” in a safer, evidence-aligned way: symptoms can be transient, can recur with titration, and should be evaluated when severe or prolonged.
Long-term considerations and the “cancer” search term
Online searches for mounjaro long-term side effects and mounjaro side effects cancer often reflect misunderstanding of class warnings. Tirzepatide labeling includes a boxed warning related to thyroid C-cell tumors observed in rodents, and it is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Human relevance of rodent findings is not established, and clinics should avoid overstating what is known. Still, the warning changes how you screen, document, and counsel. Consider adding a discrete “MTC/MEN2 screening” checkbox in your intake workflow and ensure staff know how to route related questions to a clinician.
When patients without diabetes request therapy, you may also see questions framed as “side effects for non diabetics.” From a clinic operations standpoint, the key is to document the indication, baseline metabolic risk, and shared decision-making. That documentation is distinct from the adverse-effect profile itself, which is driven by the drug’s mechanism rather than diabetes status alone.
Quick tip: Use the same symptom triage script across phone, portal, and in-person teams.
Trust cue: Clinics often require sourcing documentation before distributing brand-name injectables.
Dose Forms, Titration Documentation, and Charting
Many patients bring a “mounjaro dosage chart” from a forum or spreadsheet. Clinicians should treat these charts as non-authoritative unless they mirror the current prescribing information. The operational risk is not only clinical. It is also documentation risk: mismatched strengths in the EHR, inconsistent problem lists, and confusion between device types (pen versus vial). Standardizing how you record product form, strength, and administration day prevents errors across refills, training visits, and cross-coverage.
Reading a dosage chart safely in clinic systems
If your team fields questions like “what is the highest dose of mounjaro,” anchor the answer to current labeling rather than memory. Labels change, and different products may have different presentations. Avoid converting between mg and volume without verified concentration information. That issue comes up with searches for “mounjaro dosage chart in ml.” Many pens deliver a fixed dose and are not meant to be measured in mL by the user. If a vial presentation is used, volume-based calculations depend on the labeled concentration and your facility’s compounding and administration policies. When you do need a chart internally, build it as an EHR smart phrase that links to your most current formulary note and references the official package insert.
To keep purchasing and clinical teams aligned, it helps to catalog devices separately in inventory systems. For example, you may track Mounjaro KwikPen differently than Mounjaro Vial, even when both contain tirzepatide. If your program also supports other weekly incretin options, you may similarly separate entries for Wegovy FlexTouch 1 mg and Ozempic to reduce selection errors in ordering and charting.
In patient conversations about when to increase dose for weight loss, keep your workflow neutral: dose changes are prescriber decisions and should follow labeling and patient-specific assessment. What you can standardize is the information needed to make that decision, such as symptom burden, missed doses, and concurrent medication changes.
Injection Technique and Patient-Facing Teaching Materials
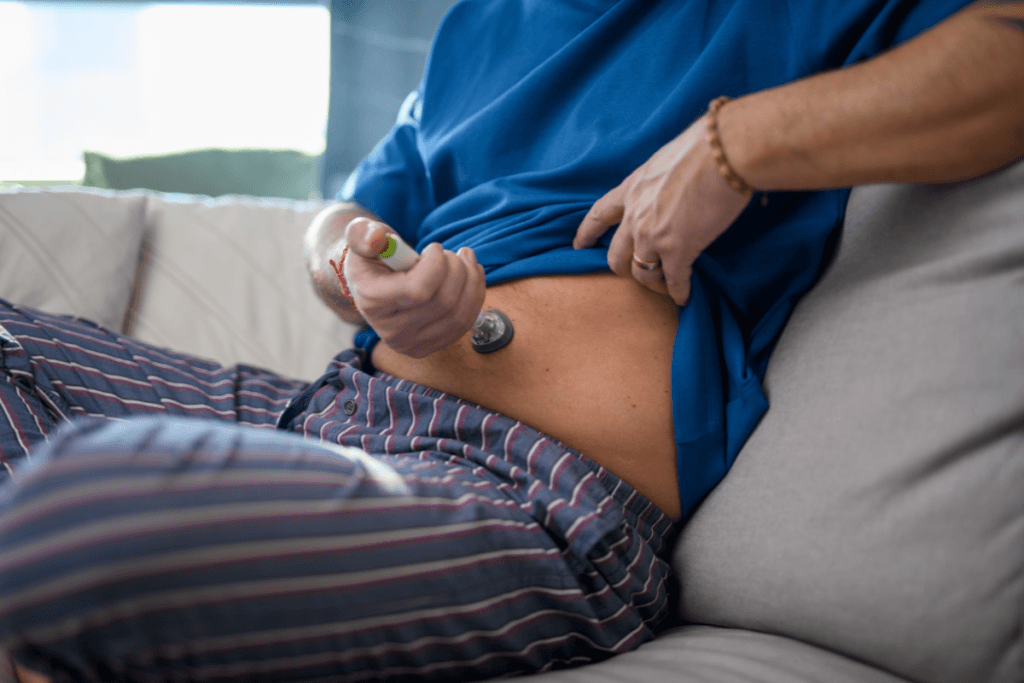
Because tirzepatide is administered subcutaneously, many program failures are actually training failures. Patients may not feel confident with needle handling, site rotation, or disposal. Some will also switch between devices over time. A brief, repeatable teaching session reduces follow-up calls and prevents unplanned discontinuation. It also gives you a clean place to document mounjaro injection education in the record, including the patient’s teach-back demonstration.
When your clinic is also supporting daily injections for other medicines, clarify differences in schedules and pen mechanics. If liraglutide remains part of your formulary, you can cross-reference device counseling for Saxenda Prefilled Pens and point staff to a broader refresher like Saxenda For Weight Loss. That kind of standardization helps when float nurses or covering clinicians step in.
Mounjaro weight loss programs also benefit from consistent non-clinical supports. Examples include a symptom diary template, a list of common food triggers, and a clear message on what information to send through portals. Those tools can be shared without giving individualized medical advice.
Common mistakes to prevent
- Skipping teach-back: no confirmation of technique.
- Unclear storage notes: staff give conflicting guidance.
- Site rotation gaps: same area used repeatedly.
- Inconsistent labeling: pen and vial confused in chart.
- Missing sharps plan: disposal not discussed.
Clinic Procurement, Verification, and Recordkeeping
Programs that scale do not rely on ad hoc purchasing. They use a defined chain of custody and consistent records, from ordering through administration. That means confirming supplier requirements, verifying licensure documentation, and ensuring your receiving staff know what to check on arrival. Storage conditions can vary by product and presentation, so align your inventory SOPs with the most current labeling and your facility’s policies.
For clinics that depend on US distribution partners, consistency in receiving and logging reduces back-and-forth when questions arise. A simple receiving checklist also supports audit readiness. It should include item verification, lot and expiration capture, and a clear process for quarantining anything that arrives with questionable labeling or damage.
Clinic workflow snapshot (high level)
Verify: confirm licensure documentation requirements and authorized recipients. Document: record device type, lot, and expiration in inventory and patient chart as applicable. Receive: inspect packaging integrity and match to the purchase record. Store: follow labeled storage conditions and separate look-alike products. Dispense/admin: document patient teaching and administration details per policy. Record: reconcile counts, track waste if applicable, and standardize adverse-event reporting pathways.
Checklist: What to standardize for your program
- Authorized staff list: who can receive.
- Inventory naming: device-specific entries.
- Lot capture: consistent EHR location.
- Patient handouts: one approved version.
- Triage script: symptom routing rules.
- Follow-up cadence: scheduled touchpoints.
- Adverse events: reporting responsibility.
Trust cue: Products are sourced through screened distribution channels to support authenticity expectations.
When patients ask how to get mounjaro for weight loss, keep your response operational and compliant. Explain your clinic’s intake steps, required documentation, and how prescribing decisions are made by licensed clinicians. Avoid informal “shopping list” guidance that could undermine safety or regulatory expectations.
For ongoing program design ideas, many teams maintain an internal resource list alongside a general Weight Loss Hub for staff training and patient education links.
How to Compare Tirzepatide With Other GLP-1 Options
Comparisons often come from patient anecdotes, including “tirzepatide reviews weight loss” threads and “before-and-after” collages. Clinics can redirect these conversations toward practical decision factors that affect workflow and adherence. These include labeled indication, dosing schedule, device handling, contraindications, and how your team monitors and documents side effects. Keep the tone neutral and align statements with labeling.
From an operational viewpoint, it also helps to compare program support needs. Some patients struggle more with nausea and meal timing, while others struggle with injection confidence or supply continuity. If your clinic offers multiple incretin options, staff education should include consistent talking points and standardized documentation fields across products. For broader context on one GLP-1 brand that patients commonly compare, see Ozempic For Weight Loss, and for addressing aesthetic concerns that can arise during weight reduction conversations, reference Ozempic Face Explained.
When patients ask whether higher doses always mean more weight loss, avoid simple yes/no framing. Instead, emphasize that tolerability, adherence, and clinical appropriateness drive outcomes, and that dose decisions follow the approved label and prescriber assessment.
Authoritative Sources
- FDA Drug Database for current U.S. labeling
- FDA Drugs guidance and safety communications
- CDC obesity overview for public health context
Further reading can help your team keep messaging consistent across therapies. Consider maintaining a shared internal folder that links to current labels, your intake template, and your approved patient education sheets.
This content is for informational purposes only and is not a substitute for professional medical advice.