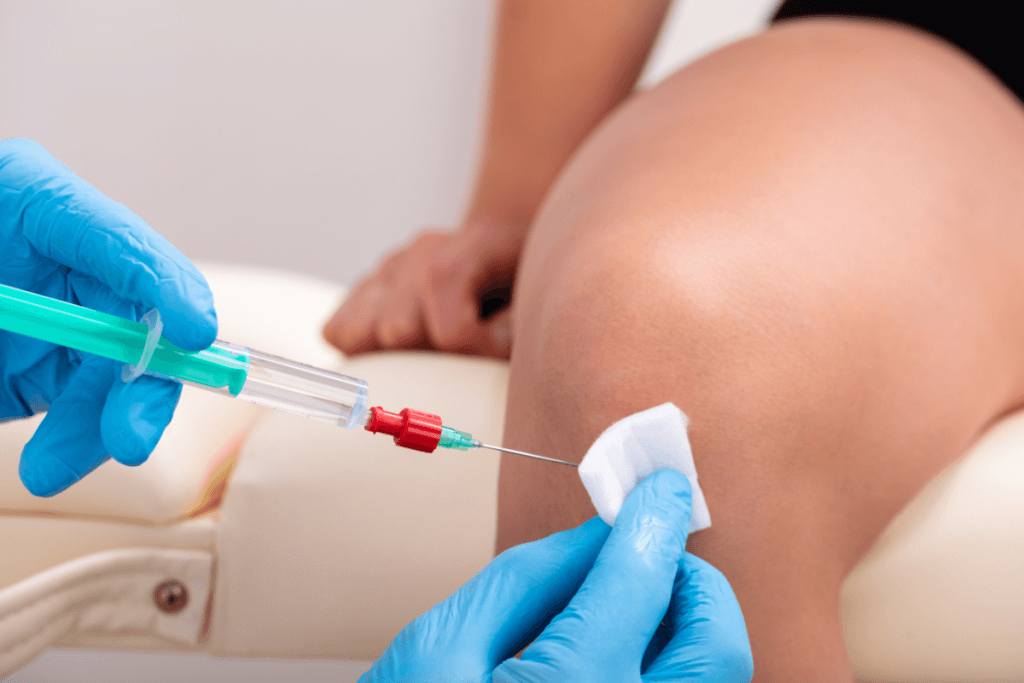
Knee osteoarthritis (wear-and-tear arthritis) is a daily operational burden for orthopedic and sports medicine clinics. Many practices use viscosupplementation (joint lubrication therapy) to support symptom management when conservative measures are insufficient. A Monovisc knee injection is one example of a single-visit hyaluronic acid (HA) option that can fit that pathway, depending on patient factors and payer policy.
For clinic teams, the practical questions are consistent. What is it (and what it is not)? What should you document around benefits and risks? How do you set expectations about timing and recovery without overpromising? And what procurement and traceability steps keep your program audit-ready?
Supply partners may verify your facility license before shipping injectables.
Key Takeaways
- Define the therapy: HA viscosupplementation, not corticosteroid treatment.
- Set expectations: onset and duration vary; avoid fixed timelines.
- Plan for flares: transient post-injection pain can occur.
- Document well: indications, consent, lot tracking, and instructions.
- Compare options: dosing schedules differ across HA and steroid products.
Monovisc knee injection: What It Is and Where It Fits
Monovisc is a hyaluronic acid–based intra-articular injection used for symptomatic knee osteoarthritis in appropriate patients. Hyaluronic acid is a naturally occurring component of synovial fluid (joint fluid). In OA, synovial fluid can lose viscosity and elasticity, which may contribute to pain with movement. This class of products is often described as “gel injections” in plain language because the material is viscoelastic.
Operationally, the most notable feature is the single-injection format. That can reduce visit burden compared with multi-injection regimens, but it does not remove the need for careful patient selection, sterile technique, and follow-up planning. For a broader clinic-facing overview of this category, the Types Of Gel Injections article is a useful orientation. Many practices also use a category hub to keep alternatives organized, such as Orthopedic Injectables.
Teams also spend time clarifying what the therapy is not. Patients often ask, “Is this a steroid?” The answer is no: viscosupplementation products are distinct from corticosteroid injections. The clinical counseling and documentation are different, and so are common expectations about onset and adverse events.
Why it matters: Clear framing reduces confusion and improves consent documentation quality.
How Viscosupplementation Works for Knee OA (High-Level)
Clinicians commonly describe HA injections as attempting to restore some of the joint’s normal lubrication and shock-absorbing properties. The proposed mechanisms include improved viscoelasticity of synovial fluid, better boundary lubrication at the cartilage surface, and modulation of local inflammatory signaling. These effects are patient-variable and difficult to predict at the individual level.
When patients ask how does Monovisc work, the simplest plain-language explanation is that it supplements the joint’s “cushioning fluid.” That said, avoid implying cartilage regrowth or structural reversal of osteoarthritis. Keep your messaging consistent with the product labeling and your clinic’s standard education materials.
Single-injection format: what changes for clinic planning
A single-visit HA option affects scheduling, inventory cadence, and prior-authorization workflows more than it changes clinical fundamentals. You still need a clean room setup, a standardized timeout, and consistent post-procedure instructions. From a documentation standpoint, single-injection products can simplify chart review because there are fewer administration dates to reconcile. However, they can complicate rescheduling when payers require a specific window, or when patients arrive with acute swelling that prompts deferral. If you manage multiple HA brands, align your intake templates so the “product, laterality, lot, and expiration” fields are identical across options.
For additional background reading that many teams share internally, see Synvisc One Vs Durolane and What Is Durolane Injection. These can help staff understand why product schedules and payer rules vary.
Side Effects, Contraindications, and “Pain Worse After Gel Injection”
Most counseling questions cluster around tolerability. Commonly discussed adverse effects for knee HA injections include localized pain, swelling, warmth, stiffness, and transient effusion (fluid buildup). Patients may describe a short-term flare where knee pain feels worse after gel injection, especially in the first days after administration. From an operations perspective, it helps to normalize that “temporary irritation can happen,” while also documenting red flags that warrant reassessment.
Potential complications are similar to other intra-articular injections: infection risk, bleeding or bruising, and post-procedure inflammatory reactions. Rarely, clinics may see significant inflammatory responses that mimic septic arthritis (“pseudoseptic” presentations). Your protocols should emphasize escalation pathways rather than reassurance-only scripting.
Products are sourced through vetted distributors to support brand authenticity.
Contraindications and precautions depend on the specific product label and patient context. In general, clinics screen for suspected joint infection, active skin infection at the injection site, and known hypersensitivity to product components. Because HA products differ in formulation and source materials, do not generalize allergy language across brands. When in doubt, defer to the official labeling and your institution’s allergy policy.
Aftercare and Recovery Time: What Clinics Commonly Document
Aftercare instructions should be consistent, written, and easy to chart. Monovisc knee injection recovery time is often described operationally as “back to routine quickly,” but that does not mean “no precautions.” Many clinicians advise avoiding high-impact loading or prolonged weight-bearing for a short period after injection, then returning to usual activity as tolerated. Align this message with your clinic’s policy and the product labeling, and avoid giving patient-specific directives in generic handouts.
When patients ask is Monovisc injection painful, set expectations that a brief needle-related discomfort is possible, and that post-injection soreness can occur. Use plain language alongside clinical terms. For example: “You may feel pressure in the joint (intra-articular pressure) and some aching afterward.” Document that the patient received written instructions and understands how to contact the clinic if symptoms escalate.
Aftercare checklist clinics often include in the chart
- Activity guidance: note temporary limits and rationale.
- Symptom monitoring: swelling, warmth, escalating pain.
- Site care: keep area clean per protocol.
- Medication list: confirm anticoagulants and allergies recorded.
- Follow-up plan: timeframe and contact method documented.
- Education provided: written handout filed in chart.
From a patient-experience angle, a consistent script can reduce repeat calls about exercise after knee gel injection. From a compliance angle, it reduces variance across clinicians and locations.
Comparing HA Options and Common Alternatives
Clinics regularly field comparison questions framed as durolane vs monovisc, monovisc vs synvisc, or monovisc vs euflexxa. Others ask about monovisc vs zilretta, which is a different category (extended-release corticosteroid), not an HA viscosupplement. Your best defense against oversimplification is a structured compare-and-document approach that focuses on regimen logistics, label indications, and payer policy rather than subjective “best product” claims.
When you summarize prior therapy, keep the wording factual. A note like “Monovisc knee injection previously administered; response variable; no complications recorded” is more durable than unqualified endorsements. For deeper context, staff may reference Monovisc Vs Synvisc and Orthovisc Vs Synvisc.
How to compare products without overpromising outcomes
Use a small set of repeatable decision factors. First, dosing schedule and visit count: single-injection versus multi-injection regimens affect staffing, room utilization, and patient travel burden. Second, formulation differences: HA products vary in molecular characteristics and cross-linking, which may affect handling and subjective feel during injection, but does not guarantee a predictable clinical response. Third, payer coverage and step-therapy rules: coverage often determines what is feasible, and documentation should reflect that reality. Finally, patient-level risk flags: history of injection reactions, infection risk concerns, and preferences around visit frequency should be recorded neutrally.
| Comparison area | What to document | Why it matters operationally |
|---|---|---|
| Regimen format | Single vs multi-visit course | Scheduling and authorization complexity |
| Class | HA viscosupplement vs corticosteroid | Different counseling and monitoring needs |
| Prior response | Patient-reported change and duration (if known) | Supports consistent re-treatment rationale |
| Adverse events | Flare, swelling, suspected reaction | Guides future product selection and consent |
For brand-specific background reading your team may encounter, see Synvisc And Synvisc One, Euflexxa Injections, and Orthovisc Knee Injections. If your clinic uses combination approaches, Cingal Injection offers context on HA products that may incorporate additional agents.
Clinic Workflow Snapshot and Procurement Checklist
Even when clinical technique is standardized, the program succeeds or fails on workflow reliability. Start by mapping your end-to-end process and defining who owns each step. A consistent process also supports audits, inventory accuracy, and patient communication. If your clinic uses a supplier such as MedWholesaleSupplies, expect a model oriented to licensed healthcare professionals and brand-name products sourced through screened channels.
Lot documentation is typically maintained for traceability and audits.
Workflow snapshot (high-level)
- Verify: patient coverage requirements and clinic eligibility.
- Document: indication, consent, laterality, and baseline status.
- Request: product selection and any required paperwork.
- Receive: inspect packaging integrity and expiration on arrival.
- Store: follow label storage conditions and segregation rules.
- Administer: aseptic process per clinic policy.
- Record: product identifier, lot, expiration, and patient response notes.
Procurement teams often keep a short checklist near receiving. Include storage verification, lot capture, and “do not use if compromised” criteria. When a single-injection HA is selected, ensure the scheduling team understands that missed appointments can disrupt authorization timing. If you stock multiple viscosupplements, avoid look-alike errors by separating bins and using barcode checks where available.
Quick tip: Keep one standardized template for all intra-articular injectables.
For reference links used by inventory teams, see the Monovisc Product Page and the Orthovisc Product Listing. Many practices also note their supplier’s US distribution footprint in continuity plans, but local receiving policies should drive your final process.
To keep charting consistent, record the product name exactly as supplied. That reduces downstream confusion when staff review Monovisc knee injection history for repeat treatment eligibility or adverse event reconciliation.
Authoritative Sources
Use primary sources to anchor your clinic materials. Product labeling is the best reference for indications, contraindications, storage conditions, and reported adverse events. Professional society guidance can help standardize which patients are considered for injection therapies and how to sequence non-operative options.
For evidence-based knee OA guidance, see the American Academy of Orthopaedic Surgeons knee OA guideline. For pharmacologic and nonpharmacologic management principles, the American College of Rheumatology clinical guidance is a helpful starting point. For regulatory context on medical products, consult the FDA medical devices resources relevant to viscosupplementation products.
Further reading can also include your internal policies on consent language, infection prevention, and documentation standards. Keep those documents aligned across clinicians, procurement, and billing teams.
This content is for informational purposes only and is not a substitute for professional medical advice.