Description
What Prevenar 13 Is and How It Works
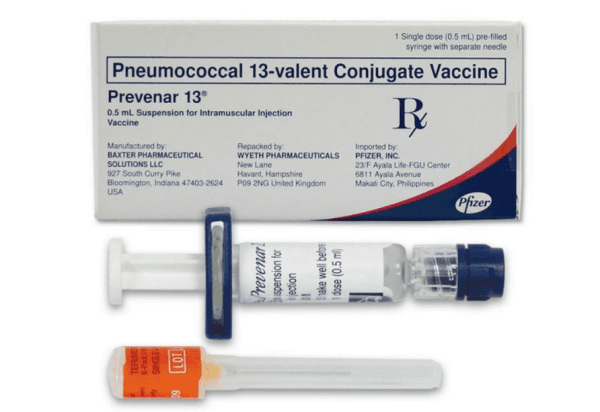
Prevenar® 13 is a pneumococcal conjugate vaccine supplied as a sterile suspension for intramuscular administration. Clinics use the Prevenar 13 vaccine in evidence-based immunization workflows to help protect eligible populations against disease caused by Streptococcus pneumoniae. For procurement planning, we support streamlined purchasing aligned with US distribution to meet routine and catch-up vaccination program needs.
Med Wholesale Supplies serves licensed clinics and healthcare professionals with authentic, brand-name medical products sourced through vetted distributors. This preparation contains 13 capsular polysaccharide antigens individually conjugated to a carrier protein, designed to help the immune system recognize targeted pneumococcal serotypes. The product is intended for administration by trained personnel following current professional guidelines and local protocols.
In clinic settings, the vaccine is typically given intramuscularly, with site selection based on age and patient factors. Teams should reference authoritative schedules for timing and spacing relative to other immunizations. Labeling provides storage and handling guidance so your staff can maintain chain-of-custody and traceability across receipt, refrigeration, preparation, and administration.
Professional Applications
Facilities integrate the PCV13 vaccine into pediatric well-visit schedules, adult risk-based vaccination, and public health outreach. It fits into primary care, internal medicine, pediatrics, pharmacy-based vaccination programs, and population health initiatives. Practices often align appointment templates, vaccine screening, and consent capture within EMR immunization modules for consistent documentation.
Group practices coordinate staff training for vaccine storage, temperature monitoring, and lot tracking. Larger organizations standardize inventory rotation, establish periodic counts, and configure recall lists for follow-up visits. To broaden category planning for your formulary, see our Pharmaceuticals category for adjunct products and related therapies that share similar cold-room handling principles.
Key Features
- 13-valent coverage: Pneumococcal 13-valent conjugate vaccine for specified serotypes.
- Clinic-ready form: Sterile suspension intended for intramuscular injection.
- Professional use: Administered by qualified healthcare personnel only.
- Traceability: Manufacturer lot and expiry visible for EMR recording.
- Regulatory grade: Produced under stringent quality and GMP standards.
- Clear labeling: Storage conditions and handling guidance on pack.
- Workflow fit: Unit-of-use presentation supports point-of-care protocols.
- Tolerability profile: Local reactions and transient fever may occur; serious hypersensitivity is rare.
- Planning support: Staff can review device needs; see How To Buy Cannulas.
Benefits in Practice
The Prevnar 13 vaccine integrates smoothly into standardized vaccination visits and community immunization days. Staff can pre-stage consent forms, screen for contraindications, and capture lot numbers at the point of care. The preparation’s consistent presentation supports training, reduces variability across providers, and helps maintain throughput during seasonal surges or outreach clinics.
For multi-service clinics, aligning biologics across departments can simplify handling and oversight. Teams planning immunology programs may also review Immunology Therapy selections. Facilities addressing cardiometabolic risk may reference our Cardiometabolic Injection listing for category planning and storage protocol parallels.
Composition & Ingredients
This class of vaccine comprises capsular polysaccharides from 13 pneumococcal serotypes, each covalently linked to a nontoxic diphtheria-derived carrier protein (CRM197). The conjugation is designed to enhance T-cell–dependent immune responses. The adjuvant system typically includes aluminum phosphate to support antigen presentation. Common excipients may include buffers, stabilizers, and isotonic agents appropriate for parenteral use.
Formulation components in a Pneumococcal conjugate vaccine PCV13 commonly include elements such as a succinate buffer, polysorbate 80, sodium chloride, and water for injection. Exact excipient lists can vary by market presentation; consult the manufacturer documentation and local labeling included with your shipment. Always review the package insert for composition, handling instructions, and any updates to formulation or contraindications.
Packaging & Supply
Clinic teams typically receive a sealed unit-of-use presentation suitable for single-patient administration, accompanied by manufacturer labeling and a package insert. Carton and primary container display lot numbers and expiry dates for accurate EMR entry and inventory rotation. This supports stock integrity during receipt, check-in, and placement into monitored refrigeration.
Sites serving mixed populations may align stock allocations for pediatric schedules and risk-based adult care, ensuring the right quantity planning for Prevnar 13 for adults where appropriate under current recommendations. For cross-department operations and appointment bundling, see our reference content such as the Contraception Implant Article to evaluate staffing and patient flow considerations relevant to injectable services.
Ordering & Logistics
Licensed accounts can sign in to view available pack configurations and request volume or contract pricing. New customers are verified for professional credentials prior to activation. After approval, your team can place replenishment requests, align lead times with clinic calendars, and designate receiving locations with monitored refrigeration.
We coordinate fulfillment windows around business hours to support check-in, temperature verification, and immediate placement into storage. Include your contact details for carrier notifications and designate a backup receiver to reduce delays. For operational planning beyond vaccines, our PDO Threads Guide offers general insights on stocking, scheduling, and patient flow that translate well to injection-based services.
Comparable Products
For clinics building immunization portfolios, consider adding a routine childhood vaccine alongside this pneumococcal line. See our listing for MMR Vaccine to support comprehensive pediatric scheduling and inventory planning across common visit templates.
Pricing & Access
Sign in to your verified account to view current pricing. Volume tiers and contract pricing are available for qualified organizations based on order frequency and aggregate purchasing. Your account representative can assist with quotations, purchasing documentation, and formulary alignment for system-wide rollout.
Availability & Substitutions
Supply can vary due to manufacturing cycles and public health demand. When inventory is constrained, we will discuss suitable substitutions within your clinical protocol and confirm acceptance with your medical leadership. We do not make restock guarantees; please plan reorder points based on your throughput and lead times.
Authoritative Sources
Pfizer – Product InformationCDC – Pneumococcal VaccinationFDA – Vaccines Overview
Ready to optimize clinic supply? Sign in to request access, plan inventory, and schedule delivery with temperature-controlled handling when required and tracked US delivery.
Frequently Asked Questions
Which clinical settings typically administer this vaccine?
Primary care, pediatrics, family medicine, and internal medicine clinics commonly administer this product during preventive visits and catch-up schedules. It is also used in pharmacy-based vaccination services and community health programs under standing orders. Occupational health, long-term care, and hospital outpatient clinics may incorporate it for eligible adults based on risk and shared decision-making. Always follow current guidance from ACIP/CDC or local authorities when integrating into clinic workflows.
How should clinics store the product before use?
Store under manufacturer-recommended refrigerated conditions and avoid freezing. Keep units in their original cartons to protect from light and to maintain traceability of lot and expiry information. Use a calibrated temperature monitoring system and document checks according to your policy. Rotate inventory first-expiring-first-out. If a temperature excursion occurs, quarantine the product and follow manufacturer and public health guidance before deciding on use.
Can it be given at the same visit as other vaccines?
Co-administration may be appropriate depending on age, immunization history, and current recommendations. Use separate syringes and injection sites when administering multiple vaccines during the same encounter. Review the latest ACIP/CDC schedules and spacing guidance, as well as the manufacturer’s insert, before developing standing orders. Document sites, lot numbers, and VIS delivery within your EMR and provide post-vaccination counseling on expected local reactions.
What documentation accompanies each unit for traceability?
Each unit typically includes a manufacturer label listing lot and expiry, along with a package insert. On receipt, record lot and expiry in your EMR or inventory system and affix any internal labels needed for tracking. Retain shipping documentation for chain-of-custody and temperature records as per your policy. During administration, capture the lot, expiry, site of injection, and the VIS date provided to the patient or caregiver.
Are needles or syringes included with the vaccine unit?
Most vaccine presentations do not include separate needles unless specified by the manufacturer. Verify your order contents and maintain an inventory of compatible syringes and needles in appropriate sizes for intramuscular administration. Ensure sharps disposal containers are available at the point of care. Review your clinic’s standard operating procedures for device selection, site preparation, and post-injection observation.
Which age groups are typically considered for PCV13 in practice?
Pediatric patients are commonly scheduled for PCV series during well-child visits. Certain adults, such as those with specific risk factors or based on shared decision-making, may also be considered under current ACIP recommendations. Check the latest guidance for indications and timing before building standing orders. When in doubt, consult the manufacturer labeling and public health resources for age-specific recommendations related to the PCV13 vaccine.
What is the recommended route of administration?
This product is intended for intramuscular injection by trained healthcare professionals. Site selection depends on age and clinical assessment. Do not administer intravenously, subcutaneously, or intradermally unless specifically directed by the labeling. Follow aseptic technique, confirm identity and consent, and observe the patient afterwards per clinic protocol. Document lot number, expiry date, injection site, and any immediate post-administration observations in the EMR.
Specifications
- Main Ingredient:
- Manufacturer: Pfizer
- Drug Class:
- Generic Name:
- Package Contents: 0.5 mL x 1 intramuscular
- Storage Requirements: Store refrigerated at 2ºC to 8ºC (36ºF to 46ºF)
- Main Usage:
Here to help
Questions about ordering, delivery or products? You can email our team here or call now at 1-800-630-9757 and be connected with your dedicated Account Manager
Related Products
NEAUVIA™ ORGANIC INTENSE LIPS
RejuvaNAD+
Related Articles
Durolane Hyaluronic Acid for Knee OA: Clinical Guide
Key Takeaways Product class: A viscosupplement (joint-lubricating injection), not a corticosteroid. Clinical use: Commonly discussed…
Osteoporosis Bone Building Drugs: Anabolic Options for Clinics
Key Takeaways Define the goal: distinguish bone-forming therapies from antiresorptives. Sequence thoughtfully: transitions can matter…
Md Ceuticals Sunscreen Clinical Guide for Clinic Selection
Key Takeaways Md ceuticals sunscreen can be assessed like any clinical sunscreen: verify claims on…
Sculptra Side Effects: What Clinics Should Screen and Track
Key Takeaways Tracking Sculptra side effects starts with clear expectations, tight documentation, and consistent follow-up.…
Hyaluronidase for Lip Filler: Clinical Workflow Essentials
Key Takeaways Hyaluronidase for lip filler is typically used to reverse hyaluronic acid gel in…
What Is Mesotherapy? Clinical Uses, Risks, and Workflow
Key Takeaways Define the technique: what is mesotherapy refers to superficial multi-injection delivery of small…
Juvederm Before And After Photos Documentation Guide For Clinics
Key Takeaways Standardize images: Control pose, lighting, lens, and distance. Document context: Record product, lot,…
Esthetician License Requirements for Med Spa Clinic Teams
Key Takeaways Verify licensure status: confirm active status with the state board. Match role to…