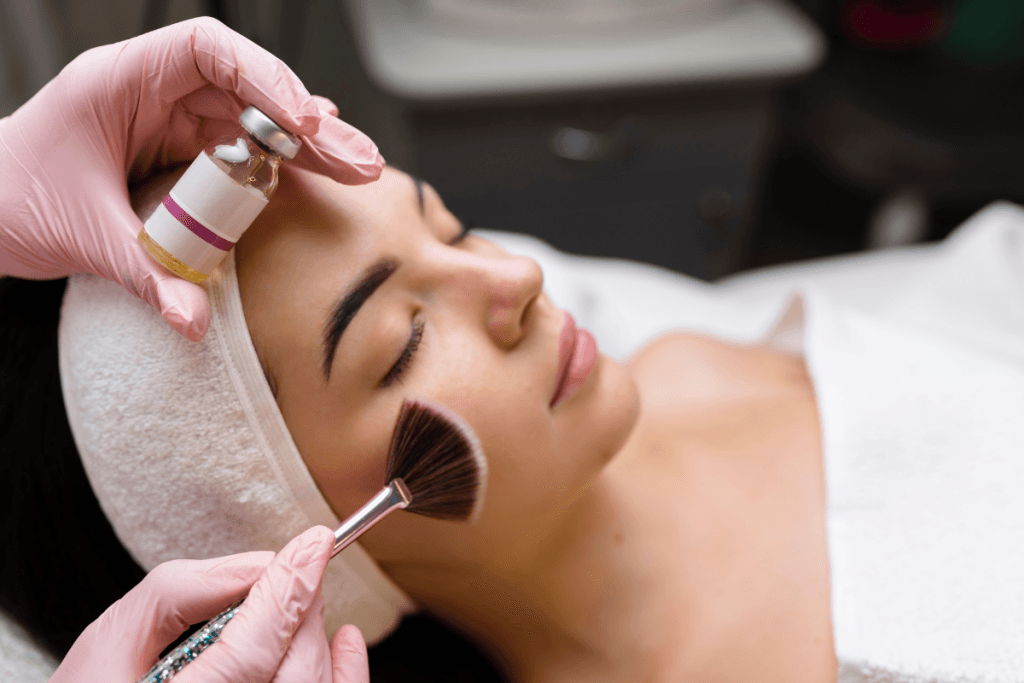
Chemical peels sit at the intersection of results, downtime, and risk control. BioRePeelCl3 is often discussed as a “biostimulating” peel option, meaning practices evaluate it for texture, tone, and acne-prone presentations, while trying to keep recovery predictable. When you order biorepeelcl3 wholesale, the operational questions matter as much as the clinical ones. You need a clear workflow for verification, staff training, documentation, and storage. You also need consistent patient selection and aftercare messaging across providers.
This guide frames BioRePeelCl3 in practical, clinic-facing terms. It focuses on mechanism concepts, common use-cases, protocol planning, and operational controls. It does not provide dosing, treatment settings, or patient-specific medical advice.
Key Takeaways
- Define your peel goals before selecting a “biostimulatory” option.
- Standardize screening, consent language, and post-procedure instructions.
- Confirm version, labeling, and IFU before stocking any peel.
- Plan spacing if combining peels with devices or injectables.
How BioRePeelCl3 Fits in a Modern Peel Portfolio
BioRePeelCl3 is commonly positioned as a professional peel with a multi-component formulation. In clinic conversations, it is often compared with classic trichloroacetic acid (TCA) peels, “no-peel” chemical approaches, and other blended acids. The key operational point is that “TCA-containing” does not automatically predict downtime or depth. Depth and tolerability depend on multiple variables, including the full formulation, contact time, skin preparation, layering choices, and patient factors.
Many clinics group peels into practical buckets: brightening for dyspigmentation (uneven pigment), texture-focused for photodamage, acne-prone support, and body-area resurfacing. If you maintain a broader peel menu, consider creating a simple internal pathway that routes patients by primary concern and risk profile, then narrows to products based on training, supply reliability, and post-care capacity. For broader context, your team can review the Anti-Aging Solutions With Chemical Peels article as a non-brand overview.
MedWholesaleSupplies supplies only verified clinics and healthcare professionals.
Why it matters: Peel outcomes are often driven by consistency, not complexity.
BioRePeelCl3 Biostimulating Peel Explained: What “Biostimulatory” Means
Clinicians often use “biostimulatory” as shorthand for approaches intended to support visible skin renewal beyond simple exfoliation. With chemical peels, that concept typically points to controlled chemical injury plus an environment that supports recovery. In practice, you still need to think in the fundamentals: barrier disruption, inflammation (as a normal response), re-epithelialization, and how that translates into texture and tone change.
When teams evaluate this category, they usually ask three questions. First, what is the intended depth and variability across skin types? Second, how predictable is post-treatment appearance and downtime for your patient population? Third, how forgiving is the protocol when different staff members deliver it? Those questions matter whether you provide BioRePeelCl3, a classic TCA peel, or a blended acid product from your broader Peels And Masks inventory.
Some clinics first encounter BioRePeelCl3 through supply planning. If you are comparing vendors or consolidating SKUs, document how you will authenticate lots, store them, and train staff. Those steps reduce variability long before you see a patient. In that context, order biorepeelcl3 wholesale decisions should be tied to a written protocol and a competency checklist, not just demand signals.
Ingredients, Formulation, and Mechanism of Action: Practical Takeaways
BioRePeelCl3 is described as a multi-ingredient peel system that includes exfoliating acids and supportive components. Exact contents, concentrations, and pH should be confirmed on the current manufacturer labeling and instructions for use (IFU). Avoid relying on secondary summaries, which can be outdated or region-specific.
At a high level, blended peel formulations aim to balance keratolysis (breaking down outer skin layers) with tolerability and predictable recovery. The clinical “feel” of a peel is influenced by more than the headline acid. Vehicle, viscosity, stabilizers, and adjunct ingredients can affect spread, stinging, and residue. For operations, that matters because it affects how long a room is occupied and what post-procedure appearance is typical in your setting.
How to Compare Mechanisms Without Overstating Claims
Mechanism discussions easily drift into marketing language. A more reliable clinic approach is to translate mechanism into controllable variables. Ask which variables your team can standardize and which will vary by operator. For example, you can standardize pre-cleanse steps, skin degreasing, timing discipline, and aftercare handouts. You cannot fully standardize individual barrier function, prior retinoid exposure, or inflammatory reactivity.
When you want to compare BioRePeelCl3 with other blended peels, consider creating a one-page internal brief with: (1) intended use-cases, (2) typical visible recovery pattern in your patients, (3) key contraindications from the IFU, and (4) “do not combine” rules based on your medical director’s policy. That framework is more actionable than debating whether one peel is “stronger.” If your practice also stocks alternatives, you may cross-reference products your team already knows, such as PRX-T33 WIQO or Ferulac Peel Plus, while keeping the comparison operational rather than promotional.
For clinics building a consistent acquisition pathway, order biorepeelcl3 wholesale planning should include a written summary of the formulation as listed on the IFU you will stock.
Patient Selection, Indications, and Downtime Expectations
Clinics commonly consider this peel category for acne-prone skin, post-acne texture, uneven tone, and photoaging concerns. When patients say “dark spots,” you still need to differentiate sun spots, post-inflammatory hyperpigmentation (PIH), and melasma, because each behaves differently. If pigment is a major driver in your patient population, keep a simple internal pathway and align it with your broader protocol set. The clinic-friendly overview in Chemical Peel For Hyperpigmentation can help staff use consistent language.
Downtime is a workflow issue. You need to set expectations using conservative, non-promissory language and define what “normal recovery” looks like for your clinic. Many practices script these expectations as: immediate erythema (redness), transient tightness, possible visible flaking, and sensitivity to sun exposure. Then they align skincare restrictions and follow-up touchpoints to that script. Your documentation should reflect what you told the patient, not only what you did.
Selection and precautions should be driven by your medical director’s policy plus the manufacturer’s contraindications. Common screening domains include: recent isotretinoin exposure, active dermatitis, infection risk (including herpes simplex history if relevant), pregnancy or breastfeeding status based on policy, and recent procedures that compromise the barrier. Use people-first language and avoid implying that one “skin type” is always suitable.
For practices offering this peel across multiple providers, a standardized intake form matters. If you order biorepeelcl3 wholesale for more than one location, align intake, consent, and aftercare documents across sites to reduce drift.
BioRePeelCl3 FND vs Body Differences That Matter in Clinic Operations
BioRePeelCl3 is discussed in variants intended for different treatment areas. Clinics often describe “FND” for face/neck/decolletage workflows and “Body” for thicker skin sites. The most important operational point is not the naming. It is whether the variant matches your intended area, staff training, and aftercare capacity. Confirm the exact indication language on the packaging and IFU you receive.
Body-area peel workflows also create different scheduling and privacy requirements. Hands, neck, and other visible areas can be high-satisfaction treatments, but they require careful expectation-setting. Patients use their hands constantly, and that can amplify perceived dryness or irritation. If hand rejuvenation is a growth area for your practice, coordinate peel protocols with your broader body-focused menu, such as the operational considerations in Aesthetic Treatments For Hand Rejuvenation.
| Operational Factor | FND Workflows (Typical) | Body Workflows (Typical) |
|---|---|---|
| Treatment areas | Commonly planned for facial regions | Often planned for thicker, larger areas |
| Room time drivers | Facial prep and post-care discussion | Area prep, draping, and aftercare logistics |
| Aftercare friction points | Cosmetic camouflage and sunscreen adherence | Friction from clothing and frequent hand use |
| Staff competency focus | Even application on contours | Consistency across larger surface areas |
In procurement terms, order biorepeelcl3 wholesale decisions should specify which variant(s) you intend to stock and why.
Products distributed for clinics are typically sourced through vetted distribution channels.
order biorepeelcl3 wholesale: Sourcing, Documentation, Storage
Wholesale procurement for professional peels is less about “getting inventory” and more about controlling inputs. Your practice should be able to show what you received, how it was stored, and that staff followed the IFU. That matters for patient safety, complaint handling, and internal quality reviews.
Start by confirming that your supplier supports professional-use verification. Many distributors will request clinic credentials or practitioner documentation before fulfilling restricted products. Build that step into onboarding for new locations or new legal entities. If your clinic team browses assortments, you can keep a short list of comparable products for reference, such as Salipeel DS or Filorga Light Peel, but your final selection should be protocol-driven.
Clinic Procurement Checklist
- Verify clinic licensure documentation is current
- Confirm exact product name and variant
- Review IFU for contraindications and handling
- Check lot number and expiry on receipt
- Document storage conditions per IFU
- Train staff on a single protocol version
- File incident and complaint reporting steps
Quick tip: Keep IFUs accessible in each treatment area.
Storage and handling should follow the manufacturer’s directions. If the IFU calls for light protection, temperature limits, or special stability considerations, document where and how you meet them. Avoid “common sense” substitutions that are not supported by the label. Also clarify who can access peels, how you track opened units, and what your clinic defines as “in-use” versus “quarantined.” Policies vary by jurisdiction and medical director preference.
Some practices choose suppliers because fulfillment aligns with reliable US logistics, but it should never replace your in-clinic controls.
MedWholesaleSupplies focuses on brand-name products intended for licensed professional settings.
Protocol Planning: Pre-Treatment Prep, Aftercare, and Maintenance
Protocol consistency is your strongest safety tool. Even when clinicians individualize care, the baseline steps should remain stable: standardized skin assessment, pre-procedure cleanse and prep, timing discipline, and documented aftercare instructions. Keep your workflow compatible with patient needs, including those who travel, work outdoors, or cannot tolerate visible flaking.
Pre-treatment preparation is usually about barrier management and reducing avoidable irritation. Many clinics use a simple policy that flags recent exfoliants, retinoids, waxing, and other procedures that can increase sensitivity. Then they define a conservative “pause” period based on clinical judgment and product IFU guidance. Avoid giving patients contradictory instructions across providers. If you use educational resources on your site, the Peels And Masks hub can support consistent staff language.
Aftercare and maintenance should be framed in plain language. Patients need to understand basic sun protection, gentle cleansing, and avoiding picking or aggressive scrubbing. They also need to know what changes are expected versus unusual. If you track outcomes, use standardized photos and consistent lighting. “Before and after” documentation is most useful when it is repeatable and audited internally.
To reduce confusion, build a simple cadence document for “how often” your clinic typically schedules peel visits, with the caveat that timing is individualized and guided by professional assessment. If you order biorepeelcl3 wholesale for a recurring protocol series, align that cadence with inventory par levels and expiration management.
Combining With Other Procedures and Layering With Peels
Combination planning is where clinics can unintentionally increase risk. The issue is often not the product, but the total barrier load. When you combine chemical exfoliation with devices, injectables, or additional peels, you should define sequencing rules and minimum separation windows in your internal policy. Those specifics should be set by your medical director and informed by the IFUs for each modality.
Microneedling is a common consideration because it intentionally creates microchannels. Layering a peel too close to microneedling can change tolerability and recovery in unpredictable ways. If your clinic offers device-based resurfacing, treat peel and device scheduling as a single plan rather than separate services. Use plain documentation: what was done, in what order, and what the patient was instructed to do afterward.
Pitfalls to Avoid When Building a Combination Menu
- Stacking irritation across multiple modalities
- Inconsistent contraindication screening across staff
- Unclear patient instructions after multi-step visits
- Skipping standardized photos and chart templates
Also consider how you educate patients about realistic outcomes. Many concerns like acne scars and melasma are chronic or multifactorial. A peel may help some aspects, but it is rarely a single-step solution. For broader trend context, share internal reading such as Non-Surgical Aesthetic Treatments For 2025 to help teams align messaging across modalities.
From a procurement angle, order biorepeelcl3 wholesale should be coordinated with the products you routinely combine in the same treatment plan, including cleansers, post-procedure barrier products, and sunscreen recommendations.
Authoritative Sources
Regulatory status and permitted claims can vary by country. When you evaluate labeling terms such as “CE marking” or professional-use classification, rely on primary sources and your local regulatory guidance. Avoid copying claims from reseller listings or social posts. If you need to validate whether a device or cosmetic product must meet certain requirements, confirm with the relevant authority in your jurisdiction.
These neutral resources can support team education on peel basics and regulatory concepts:
- American Academy of Dermatology overview of chemical peels
- U.S. FDA cosmetics regulatory information
- European Commission overview of medical devices and CE marking
Further reading can also help standardize staff language and expectations. Review your clinic’s peel protocols annually, and update them when IFUs change or new staff join.
This content is for informational purposes only and is not a substitute for professional medical advice.