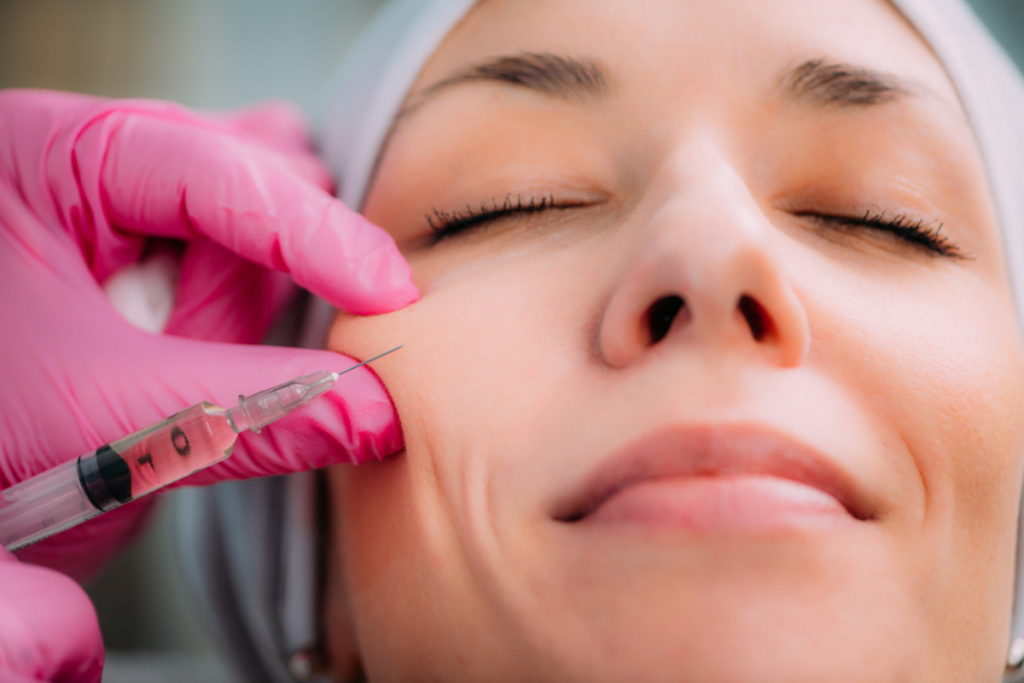
Clinics often compare teosyal vs juvederm when standardizing hyaluronic acid (HA) dermal filler options. The challenge is not the brand name. It is aligning product families, labeling, and rheology language with your common indications and risk controls.
This guide frames the comparison as a clinic workflow problem. You will see what to verify on official labeling, how to discuss common treatment areas, and how to interpret “reviews” and before-and-after marketing without over-weighting anecdotes.
Supplies are provided only to verified clinics and healthcare professionals.
Key Takeaways
- Compare by use-case: map areas, injector comfort, and complication plans.
- Verify label details: indications, contraindications, and any included anesthetic.
- Standardize documentation: lot tracking, consent language, and photo protocols.
- Don’t overread “reviews”: prioritize controlled training and published safety guidance.
- Audit sourcing steps: authenticity, chain-of-custody, and receiving checks.
Teosyal vs Juvederm: How to Compare in Practice
Most clinics make better decisions when they start with “what problem are we solving?” rather than “which brand is better?” In day-to-day practice, you are balancing tissue plane, desired projection, edema risk, and your team’s comfort with a given product family. You are also balancing operations: training requirements, documentation, and the ability to maintain consistent inventory.
Begin with a simple map of your top requests: lips, midface volume, nasolabial folds (smile lines), marionette lines, and under-eye hollows. Then overlay your preferred injection approaches and your complication response plan. A comparison becomes clearer when each candidate has a defined role, rather than being treated as interchangeable.
Why it matters: Standardization reduces variability in both results and documentation quality.
Start With Your Use-Case, Not The Brand
A practical comparison looks like an internal “formulary” discussion. You list which fillers are approved and stocked, what each is used for in your practice, and which clinicians can use them. This approach also improves continuity when staff turnover happens or when a new injector joins. If you need a quick refresher on the broader landscape, the Types Of Dermal Fillers overview can help you place HA fillers alongside other biostimulatory (collagen-stimulating) options.
At this stage, keep your language neutral. Avoid promising longevity or “best” outcomes. Instead, document what is verifiable: label indications, product family characteristics described by the manufacturer, and your own clinic’s training standards. For clinics building a curated set of options, it can also help to browse a hub view like the Dermal Fillers Category to see how products are grouped operationally.
How To Compare Crosslinking Claims
Marketing often highlights proprietary crosslinking or manufacturing terms. These terms can be useful, but only if you connect them to clinical handling and risk controls. Start by collecting the instructions for use (IFU) and any official training materials. Then translate each claim into a question your team can answer consistently. For example: Does the product family include multiple gels for different planes and areas? What injection depth language is used on labeling? What warnings are emphasized for high-risk anatomy?
Keep “technology” terms as descriptors, not as proof of superiority. If you are comparing families discussed online—such as “teosyal rha vs juvederm vycross”—treat that as a prompt to verify which specific SKUs your clinic is evaluating, and what each SKU is approved to do in your jurisdiction. Crosslinking language does not replace a clear plan for patient selection, photography standards, and complication readiness.
Formulation Language and Product-Family Differences
Both brands are HA-based, but they are organized into distinct product families. Those families may differ in gel behavior, included anesthetic options, and how manufacturers describe intended treatment depth. In procurement terms, this means you are not comparing two single products. You are comparing portfolios that can be configured for your service menu.
When teams evaluate teosyal vs juvederm, confusion often comes from mixing family names with individual SKUs. Create a one-page reference that lists the exact product names you stock, their labeled indications, and the anatomical areas you commonly treat with each. For internal education, you may also find it useful to review brand-specific background pieces like Teosyal Filler Overview and Juvederm Treatments Explained, then reconcile them with official labeling.
Inventory is brand-name and intended for professional clinical use.
| Comparison Dimension | What To Check | Why It Helps Clinically |
|---|---|---|
| Portfolio structure | Family names vs individual SKUs | Prevents “apples-to-oranges” substitutions |
| Labeling language | Indications, depth, and technique notes | Supports consistent charting and consent |
| Included components | HA source, any anesthetic, excipients | Clarifies allergy and counseling considerations |
| Handling profile | Extrusion force, swelling tendency (as observed) | Improves injector-to-injector consistency |
| Risk controls | High-risk area warnings and contraindications | Aligns with complication prevention protocols |
Operationally, consider whether your suppliers can support consistent lots and documentation for the specific SKUs you rely on. If you carry multiple HA lines, you may also compare against other common families. Some clinics triangulate choices with comparisons like teosyal vs restylane or stylage filler vs teosyal, especially when building a tiered menu for different textures and areas.
Treatment-Area Considerations: Lips to Tear Troughs
Most “brand vs brand” discussions actually become “area vs area” discussions. That is why your internal comparison should follow your appointment mix. Lip augmentation, midface contouring, and under-eye hollow correction each have different tolerance for swelling, lumpiness, and visibility of product edges. Patient perception also varies by area, since small irregularities are more noticeable around the mouth and eyes.
In practice, teosyal vs juvederm conversations frequently center on lips. You may hear patients ask about “teosyal filler lips” or “teosyal kiss vs juvederm,” often based on social posts. Translate that into a clinic task: confirm which specific lip-oriented SKUs are on label for your market, define your photography angles, and standardize your post-procedure guidance language. Keep your promises conservative and tied to what you can control.
Common Area Requests and Decision Points
Lips: Plan for swelling, asymmetry, and short-term texture changes. Document baseline lip competence, prior filler history, and any history of herpes labialis (cold sores), per your clinic policies. If your practice uses a lip-focused SKU such as a “kiss” product name, treat it as a specific tool, not a guarantee of shape. Also decide whether you will stock more than one lip gel for different goals, or standardize to one for training simplicity.
Cheeks and midface: Define whether your clinic prioritizes lift, contour, or anterior projection, and align that with the manufacturer’s treatment-area language. If you want more context on midface planning, the article Juvederm Voluma Lift And Contour is a useful orientation piece, but still confirm the specific product’s local approval and IFU.
Nasolabial folds and marionette lines: These areas often involve managing shadowing and dynamic movement. Standardize how you describe expected softening rather than “erasing,” and document dental factors when relevant. Many clinics treat these as part of a perioral plan rather than isolated lines.
Under eyes: Tear trough (under-eye hollow) treatments are higher-visibility and can be higher-risk. If your team uses a dedicated under-eye SKU from another line, it can be helpful to compare labeling approaches side-by-side. For example, some clinics will review a product like Restylane Eyelight to understand how manufacturers communicate under-eye use, then decide what level of specialization they need in their own portfolio.
Finally, keep room for individualized planning. “One filler for everything” can simplify inventory, but it can also create off-label pressure. Your best operational compromise may be a small set of well-understood SKUs with clear internal rules.
Longevity, Results, and Interpreting “Reviews”
Teams often get asked, “teosyal filler how long does it last?” or whether one brand “lasts longer.” For HA fillers, duration is typically discussed in months, but it varies widely by area, injection plane, product selection, and patient factors. Your safest posture is to describe ranges conceptually, document what was used, and plan follow-up based on clinical assessment rather than marketing timelines.
When clinicians search teosyal vs juvederm longevity, the most useful takeaway is not a single number. It is which variables you can standardize. For example, you can standardize product choice by area, photography conditions, and follow-up scheduling windows. You can also standardize how you record touch-ups, so your clinic has real internal data.
Online content adds noise. You will see “teosyal filler reviews,” “teosyal lip filler reviews,” and forums like “teosyal vs juvederm reddit.” Treat these as signals about what patients notice (swelling, texture, downtime), not as evidence of comparative performance. Before-and-after photos can be educational, but they are sensitive to lighting, pose, and timing. If your staff needs a framework for discussing images with patients, the article Juvederm Before And After is a helpful starting point for setting photo expectations and documentation standards.
From an operations standpoint, decide what your clinic will and will not say. Avoid absolute statements about results. Focus on process: careful assessment, consent, and follow-up. Then use your own outcomes tracking to refine your portfolio over time.
Safety and Side Effects: What to Screen For
No HA filler is “risk-free.” Your comparison should emphasize adverse event prevention and response readiness. Common transient effects include bruising, swelling, and tenderness. More serious complications can include infection, nodules, and vascular occlusion. High-risk anatomy and injection depth vary by area, so safety planning must be area-specific, not brand-specific.
In many clinics, teosyal vs juvederm safety questions come up most in under-eye and perioral work. Use these questions to tighten your processes. Confirm contraindications on labeling, document relevant history, and ensure your clinicians have current training on anatomy and emergency response. If your practice uses hyaluronidase to dissolve HA filler, maintain protocols for access, documentation, and escalation pathways based on local guidance and clinician judgment.
Also separate “ingredient” curiosity from clinically relevant screening. Patients may ask about “teosyal vs juvederm ingredients” or whether one has lidocaine. The right response is to confirm the specific SKU’s labeling, document sensitivities, and avoid assuming formulations are identical across a brand portfolio.
For teams building a broader injectable menu, you may also compare safety expectations across product classes. For example, Sculptra Vs Juvederm helps frame how HA fillers differ from biostimulatory products in counseling and follow-up, without turning the decision into a popularity contest.
Clinic Workflow Snapshot and Procurement Checklist
Once you have a clinical map, lock in the operational steps that keep care consistent. This includes credential verification, product verification at receiving, and traceability in the medical record. Many practices treat fillers like implantable devices for documentation discipline, even when regulations differ by region.
Clinics that serve a professional market often need sourcing that supports documentation and authenticity review. MedWholesaleSupplies works with screened distribution partners to support product verification and traceability.
If your team relies on US distribution, build receiving steps that match your internal quality system. Separate “what the supplier does” from “what your clinic must document.” Policies vary by organization and jurisdiction, so keep the checklist adaptable.
Quick tip: Create a single intake form for filler history across all brands.
Workflow Snapshot
- Verify account credentials and scope of use
- Confirm SKU, lot, and expiration on receipt
- Document storage requirements per labeling
- Assign inventory to treatment rooms securely
- Record product identifiers in the patient chart
- Capture standardized photos when applicable
- Log adverse events and follow-up outcomes
Procurement and Documentation Checklist
- SKU list control: restrict substitutions without clinical review.
- Lot traceability: record lot/exp in inventory and chart.
- Receiving inspection: check packaging integrity and labeling.
- Storage log: follow manufacturer conditions and monitor deviations.
- Consent language: align with area risks and off-label boundaries.
- Training file: document injector training and refreshers.
- Incident pathway: define escalation and documentation steps.
When teams compare teosyal vs juvederm for operational fit, small details matter. Consider how many SKUs you truly need, and whether your staff can stay proficient with each one. If you want to sanity-check the breadth of another HA portfolio, resources like Restylane Treatment Guide can help you think in “family + indication” terms.
To keep purchasing conversations consistent, procurement teams may reference specific catalog entries for internal alignment, such as Teosyal RHA, Teosyal Puresense, or Juvederm Ultra. Use these as identifiers for your ordering and lot tracking, not as a substitute for IFU review.
Authoritative Sources
For safety and regulatory clarity, rely on primary sources first. Manufacturer labeling and IFUs set the baseline for indications, contraindications, and handling requirements. Professional society education can then help you standardize complication prevention and documentation habits across injectors.
If you are building internal SOPs, use these references to support training and patient-facing consent language. They are also useful when auditing your own adverse event reporting workflow. Keep your sourcing and documentation steps aligned with applicable regulations and your medical director’s policies.
- FDA Dermal Fillers (Soft Tissue Fillers)
- FDA Dermal Filler Safety Facts
- American Academy of Dermatology: Dermal Fillers
Further reading inside your team can include structured brand comparisons, such as Juvederm Ultra 4 Vs Restylane Lyft, to refine how you document “like-for-like” substitutions. The goal is a small, well-controlled set of fillers, clear documentation, and consistent follow-up practices.
This content is for informational purposes only and is not a substitute for professional medical advice.