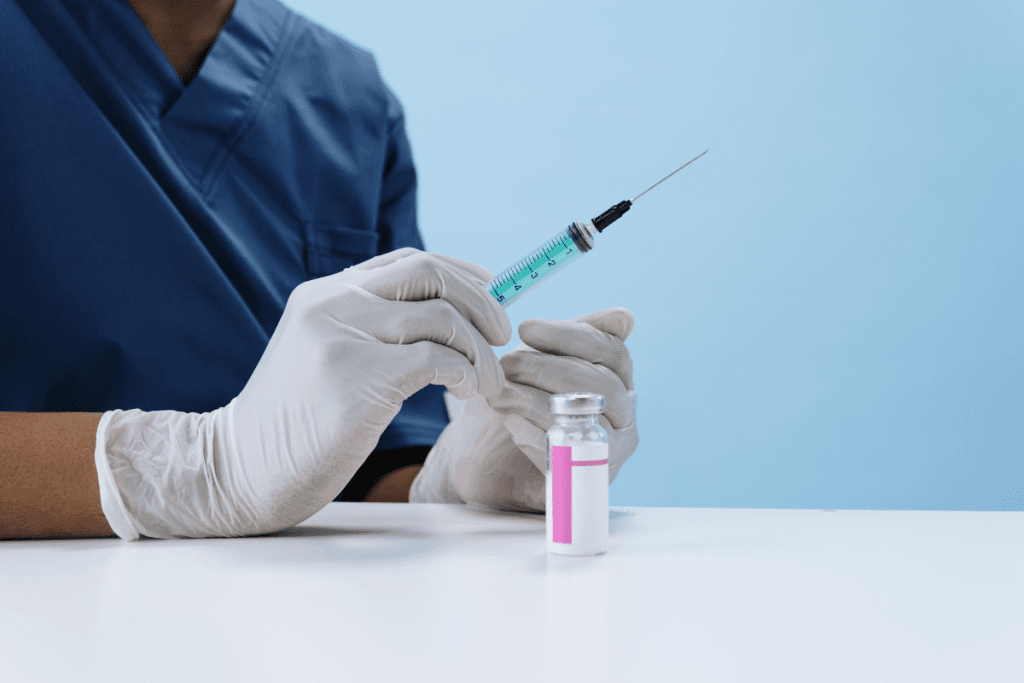
Interest in Sunekos treatment continues to grow in aesthetics, especially for “skin quality” concerns. Clinics see demand for hydration, texture, and fine-line support. At the same time, teams need a clear, repeatable way to screen candidates, set expectations, and document injectable services. This guide focuses on clinic-facing decisions, not consumer marketing.
Use it as an operational briefing for consults, consent, photography, and supply chain controls.
Key Takeaways
- Define goals: hydration, texture, laxity, or delicate areas.
- Standardize consult documentation and photo capture.
- Plan for expected injection-site reactions and follow-up.
- Align product selection with labeling and staff training.
- Maintain traceability: lot, expiry, and administration records.
Sunekos treatment: Clinical Overview and Fit
Sunekos is commonly discussed as an injectable skin-rejuvenation option positioned between classic mesotherapy and hyaluronic acid (HA) “skin boosters.” In day-to-day clinic language, patients may call it a “collagen stimulator” or “under-eye skin booster.” Your team can keep discussions more precise. Frame it as an injectable approach that may support dermal quality, while results and indications depend on local labeling, technique, and patient factors.
For product context, keep a reference list of the lines your clinic stocks or evaluates, such as Sunekos. If you offer comparable injectable categories, it can help to align your consult language with broader educational resources on Skin Boosters Injections so staff use consistent terms.
Supplier accounts are typically limited to licensed healthcare professionals.
Where It Fits vs Fillers and Devices
Patients often confuse “skin boosters” with volumizing dermal fillers. Your intake forms can separate goals into volume restoration, contour change, and surface quality. That distinction reduces mismatched expectations and complaint risk. Devices and resurfacing procedures can also overlap with skin-quality goals, but they carry different downtime patterns and consent language. For clinics offering multiple modalities, a simple decision tree can prevent “one-size-fits-all” scheduling and aftercare instructions.
Why it matters: Clear goal-setting reduces revision requests and inconsistent before-and-after photography.
Mechanism, Ingredients, and Expected Tissue Effects
From a high-level mechanism perspective, many clinics describe Sunekos as combining hyaluronic acid with amino acids, aiming to support the extracellular matrix (the skin’s structural “scaffold”). This concept is used to explain why some patients perceive changes in hydration, texture, or fine lines rather than dramatic shape changes. Keep the explanation cautious. Use “may” language and avoid promising a specific level of collagen change unless supported by official labeling in your market.
When patients ask about Sunekos ingredients, it helps to offer a plain-language translation. HA is often described as a hydrating gel that binds water. Amino acids are commonly explained as building blocks used in normal tissue processes. The exact formulation details, sterility statements, and intended use should always come from the product’s instructions for use (IFU) and your jurisdiction’s regulatory framework.
Clinics that already provide mesotherapy-style services may want to align protocols with existing training and patient education. For a refresher on category-level concepts, see Benefits Of Mesotherapy and your internal hub for the Mesotherapy Product Category.
Set Expectations for a Results Timeline
“When will I see a change?” is the most common consult question. Most clinics answer using a range, because perception varies by baseline skin quality, hydration, and photo conditions. Avoid over-precise timelines in writing. Instead, document what you will measure. Examples include standardized photos, a short patient-reported outcome scale, and a consistent follow-up window. This makes “Sunekos results timeline” discussions more objective, even when changes are subtle.
Candidate Selection, Contraindications, and Risk Discussion
A structured intake protects your clinic and your patients. Your screening should cover medical history, prior injectable reactions, anticoagulant and antiplatelet use, active dermatologic disease, and current infections. For contraindications, rely on local labeling and your medical director’s policies. Many injectable products share common exclusions, such as hypersensitivity to components or active infection at the injection site.
When you document Sunekos treatment, record the problem list you are addressing and the rationale for product choice. Include prior procedures, previous response patterns, and any risks reviewed. Keep consent language aligned with injectable procedure norms, including the possibility of bruising, swelling, redness, tenderness, and temporary unevenness. Also document rare but serious complications that apply to injections in general, and the clinic’s escalation pathway.
Under-Eye Use: Risk and Technique Sensitivity
Interest in Sunekos for under eyes is often driven by concerns about crepey skin, fine lines, or a tired appearance. The periorbital area is also unforgiving. Small changes can look uneven under overhead lighting. Bruising and edema can be more visible, and patient anxiety is often higher. Your workflow should reflect that reality. Use tighter photography standards, a clear post-procedure contact plan, and an explicit discussion of what “normal” healing looks like. If you publish outcomes, keep “Sunekos before and after” images consistent in angle, expression, and lighting.
Inventory is sourced through vetted distribution partners.
Planning Sessions: Protocol Logic and Documentation
Clinics commonly build protocols around a short initial series followed by maintenance. The specifics vary, so your best anchor is the IFU plus your clinician training pathway. Patients may ask about “Sunekos session frequency” or how long results last. Keep responses conservative. Discuss that longevity depends on baseline skin quality, lifestyle factors, and the modality mix used in the practice.
Product naming can add confusion at the consult desk. Questions like “Sunekos 200 vs 1200” often reflect patient reading rather than clinical need. Operationally, treat variant selection as a labeling and training issue. Ensure staff can explain, in neutral terms, that different versions may be intended for different areas or tissue goals. Where relevant, maintain a crosswalk in your protocol binder that maps variants to your approved use cases and consent templates.
When you evaluate adjacent options, keep your sourcing and category education aligned. Your team may find it useful to compare how patients perceive mesotherapy-type injections across services, using resources like Mesotherapy Injections Overview and the Mesotherapy Articles Hub.
Quick tip: Store current IFUs with your consent forms and photo standards.
Clinic Workflow Checklist (Non-Clinical)
- Verify credentials: licensed account and prescriber oversight.
- Confirm labeling: intended use and contraindications on file.
- Document traceability: lot, expiry, and patient record linkage.
- Standardize photos: same camera, lighting, and angles.
- Template consent: expected reactions and escalation contacts.
- Schedule follow-up: consistent window for assessment.
- Track outcomes: brief scale plus clinician notes.
If your practice carries multiple product lines, keep internal references distinct. For example, if you stock adjacent variants such as Sunekos Performa, define how that differs in your internal protocols. For multi-site practices, reliable US logistics can simplify stock standardization, but policies still vary by clinic.
Downtime, Recovery Time, and Aftercare Operations
Most clinics describe Sunekos downtime as “social downtime” driven by injection-site changes. Common expectations include redness, swelling, tenderness, or bruising. Set this in writing, and match it to your scheduling practices. For example, avoid stacking high-visibility social events next to injection appointments when patients have a history of bruising. For “Sunekos recovery time,” emphasize that visible changes can differ by site, technique, and patient factors.
Aftercare instructions should be consistent across injectables unless the IFU specifies otherwise. Keep instructions operational and observable. Examples include how to contact the clinic, what photos to send if concerned, and how to manage appointment rescheduling if visible bruising persists. If you run mixed-modality programs, cross-check aftercare language to avoid contradictions between injectables and devices.
For category-level counseling on combining or sequencing treatments, your staff may benefit from a neutral comparison of modalities, such as Mesotherapy Vs Microneedling.
Common Pitfalls to Avoid
- Inconsistent photos: different lighting or facial expression.
- Vague outcomes: no defined assessment measures.
- Overpromising: fixed timelines or guaranteed texture change.
- Thin consent: missing rare but serious injection risks.
- Poor traceability: lot numbers not linked to the chart.
How to Compare Common Skin Boosters
Clinicians and patients frequently search “Sunekos vs Profhilo vs Jalupro” to decide between product families. In practice, the comparison is less about online rankings and more about mechanism framing, injection technique familiarity, and your clinic’s ability to standardize protocols. Avoid claiming superiority without label-supported evidence. Instead, document the decision factors you used for that patient and goal.
A practical way to handle “Sunekos reviews” is to separate marketing language from measurable outcomes. Encourage your team to collect consistent satisfaction ratings, then review them by indication category (under-eye, fine lines, acne scarring) and by injector. This makes “Sunekos treatment reviews” actionable for training and quality assurance, rather than anecdotal.
Brand-name stock may include traceable lot documentation.
| Decision Factor | Sunekos | Profhilo | Jalupro |
|---|---|---|---|
| Primary consult framing | Skin quality and delicate areas | Hydration and tissue quality | Skin quality and supportive ingredients |
| What to verify in labeling | Variant intent and allowed sites | Indications and technique requirements | Formulation variant and allowed sites |
| Documentation emphasis | Photos and subtle texture metrics | Baseline hydration/elasticity notes | Goal definition and follow-up cadence |
| Training considerations | Area-specific technique sensitivity | Product-specific placement approach | Variant-based technique differences |
Keep deeper reading available for staff who counsel across product families, including Profhilo Injections Overview and Science Behind Jalupro. If you maintain product reference sheets, link out internally to items your procurement team may carry, such as Profhilo HL and Jalupro Young Eye. For trend context without changing clinical standards, you can also monitor the Beauty Trends Category.
Authoritative Sources
Because labeling and regulatory status can differ by country, keep your primary reference set local. For procedure safety, anchor training to injection-safety standards and your clinic’s adverse event response pathway. Where evidence is uncertain, document your rationale and stay within approved use.
The sources below are useful for general injectable safety and complication awareness. They are not substitutes for product-specific IFUs.
In summary, treat skin-rejuvenation injectables as a system. Outcomes improve when consult language, photo standards, follow-up windows, and traceability are consistent. Build your protocols so they are auditable and easy to train.
Further reading: review your modality mix, then update consent and documentation templates to match.
This content is for informational purposes only and is not a substitute for professional medical advice.