Knee osteoarthritis care often includes injections, therapy, and activity planning. When teams ask where certain injectables fit, the first step is clarifying what is synvisc in practical, clinic-facing terms. This guide focuses on how viscosupplementation (joint-lubricating injection) products are described, handled, and discussed in a licensed setting.
It is written for clinicians, practice managers, and procurement staff. The goal is consistent counseling, documentation, and product selection discussions. Always defer to the product’s official labeling and your facility policies.
Key Takeaways
- Viscosupplementation is a localized, intra-articular option for knee OA.
- Hylan-based gels are hyaluronan derivatives with viscoelastic properties.
- Operational fit depends on visit cadence, storage, and lot traceability.
- Aftercare messaging should cover expected soreness and escalation signs.
For broader navigation, you can browse related options in Orthopedic Injectables and align selection with your standard pathway.
What Is Synvisc in Knee OA Care Planning
In clinic shorthand, Synvisc refers to a viscosupplementation product family used for knee osteoarthritis. It is administered intra-articularly (into the joint). The intent is to supplement synovial fluid mechanics, which can be disrupted in degenerative joint disease. In day-to-day operations, it is often discussed as a “gel injection” because of its viscoelastic texture.
From a workflow perspective, this category sits between conservative therapy and procedural interventions. Many practices place it in a stepped pathway that also includes weight-bearing optimization, physical therapy, bracing, and analgesic strategies. Eligibility decisions are clinical and policy-driven. They vary by payer rules, imaging requirements, prior therapy documentation, and local protocols.
If you need deeper brand-to-brand framing for staff education, see Comparing Synvisc And Synvisc-One. For product-page reference during procurement, the site also lists Synvisc Classic Prefilled Syringes as one common format.
What tends to matter most operationally is not the marketing name. It is the formulation class, the labeled use, and how the clinic supports consistent administration and follow-up.
Material Basics and How Viscoelastic Gels Work
Teams commonly ask whether these products are “just hyaluronic acid.” In broad terms, many viscosupplements are based on hyaluronan (also called hyaluronic acid), a naturally occurring component of synovial fluid. Hylan G-F 20 is a modified hyaluronan derivative with higher viscoelasticity than native hyaluronan in many formulations. That material difference is the foundation for how the product is described in labeling and training resources.
What it is made of (and why sourcing questions come up)
When staff field questions like “what is synvisc made of,” it helps to stay close to label language. The key points are that it is a hyaluronan-derived gel and that some formulations may be sourced from avian tissue. That origin is relevant for allergy screening workflows and for documentation in practices that track biologic-source sensitivities. If your intake forms do not capture this, consider updating your pre-injection checklist.
Why it matters: Clear material explanations reduce avoidable day-of-procedure cancellations.
MedWholesaleSupplies supports licensed clinical customers with brand-name medical products sourced through vetted distributors.
Mechanism of action (high level, non-prescriptive)
When clinicians explain the synvisc mechanism of action, a conservative framing works best. These gels are intended to supplement the lubricating and shock-absorbing properties of synovial fluid. The effect is local to the joint space. Patients may describe improvements in stiffness, function, or pain interference, but response varies widely. It is reasonable to set expectations that onset and durability are not immediate for every patient, and that outcomes are influenced by disease severity, activity load, and coexisting pathology.
Operationally, you can standardize education by using one consistent “what it does” script, then deferring to the prescriber for individualized counseling.
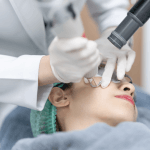
Administration Pathway: Intake, Prep, and Procedure Overview
Clinic teams benefit from a shared map of the synvisc injection procedure, even when different clinicians have different techniques. Standardization reduces friction in scheduling, rooming, and documentation. It also improves patient handoffs, especially when follow-ups occur with different providers.
Pre-visit checks your staff can standardize
Before the appointment, confirm documentation requirements and basic safety screens. Many practices verify the target joint, laterality, prior response to injections, and current infection status. They also confirm any product-specific considerations listed in labeling. From an operational standpoint, create a single-page intake addendum that staff can reuse across all viscosupplement brands.
- Laterality confirmed + documented
- Relevant allergies reviewed
- Recent procedures noted
- Anticoagulant status recorded
- Consent and education logged
For an operations-oriented discussion of scheduling and repeat courses, see Timing And Frequency Of Synvisc Injections. Keep in mind that any interval decisions must follow labeling and clinician judgment.
Procedure-day flow (high level)
Most clinics use a consistent sterile field, skin antisepsis, and standardized documentation fields. Depending on patient anatomy and clinician preference, imaging guidance may be used. Some workflows include assessment for effusion and, when clinically appropriate, aspiration before injection. From a compliance perspective, the key is capturing the product identifier, lot number, expiration date, laterality, and immediate tolerance in the chart.
At the supply level, some practices maintain separate bins for “knee OA viscosupplements” versus “intra-articular steroids” to reduce selection errors.
Aftercare Messaging and Expected Course in the First Days
Patients often ask, “what should i do after synvisc injection.” Your clinic can reduce follow-up calls by giving clear, standardized post-procedure instructions that match your prescriber’s preferences. Keep the message practical: activity modification for a short period, symptom monitoring, and when to contact the clinic. Avoid overpromising timing or degree of relief.
It also helps to normalize mild, short-lived discomfort. Transient soreness, warmth, or swelling can occur after intra-articular injections. Many clinics advise patients to avoid unusually heavy load on the joint immediately after the procedure, then return to baseline activity as directed by their clinician. Written instructions should match what staff say on the phone.
The synvisc effectiveness timeline is variable. Some patients report gradual change over days to weeks, while others do not perceive benefit. Setting a consistent “monitoring window” for follow-up calls helps your team separate expected post-injection symptoms from events that warrant assessment.
- Immediate soreness explained in advance
- Simple activity guidance provided
- Contact pathway clearly listed
- Follow-up plan scheduled
- Documentation placed in chart
For clinics seeing “knee pain worse after gel injection” complaints, the most useful operational tool is a triage script. It should capture symptom onset, swelling, fever, redness, weight-bearing tolerance, and any systemic symptoms, then route to the clinician according to your protocol.
Safety Profile, Side Effects, and Patient Questions
In staff training, emphasize that synvisc side effects are usually localized. Many reported events relate to transient injection-site or joint symptoms. This makes counseling and triage largely a matter of pattern recognition and documentation quality. Your team should record onset, severity, and associated signs, because that data guides next-step decisions and potential reporting.
Questions like “how long do synvisc side effects last” are common. A conservative answer is that minor post-injection symptoms are often short-lived, but duration varies. In contrast, severe knee pain after synvisc injection, marked swelling, fever, spreading redness, or inability to bear weight should be treated as an urgent clinical concern under your local protocol.
Patients also ask “does synvisc cause weight gain.” Because viscosupplements are localized joint injections rather than systemic weight-modifying therapies, there is no widely accepted mechanism for direct weight gain. If weight changes occur, they are more often explained by activity limitation, diet changes, or other medications. Document the concern and route it for clinician review when needed.
MedWholesaleSupplies limits access to verified healthcare professionals and licensed clinics.
For additional context on severity and “bone-on-bone” discussions, refer readers to Synvisc For Severe Osteoarthritis, while keeping your own counseling aligned to labeling and clinical assessment.
Comparing Options: Presentation, Alternatives, and Decision Factors
Many practices compare synvisc vs synvisc-one because administration burden affects scheduling and follow-up. A multi-visit series can create access challenges, while a single-visit presentation can simplify capacity planning. That tradeoff is operational, not purely clinical, and it should be discussed alongside payer rules and clinician preference.
For staff education, it helps to separate “brand” from “category.” Most comparisons are really about gel type (crosslinked vs non-crosslinked), visit cadence, syringe presentation, and prior response history. Patients may also bring synvisc-one injection reviews into the conversation. A good practice is to acknowledge anecdotal reviews, then redirect to risks, benefits, and realistic expectations.
| Comparison factor | Viscosupplement gel options | Intra-articular corticosteroid options |
|---|---|---|
| Primary goal | Support joint fluid mechanics and lubrication | Reduce inflammatory pain flare in some cases |
| Visit planning | May be single-visit or series formats | Often single-visit injections |
| Common short-term issues | Post-injection soreness or swelling can occur | Post-injection flare can occur |
| Documentation focus | Product identifiers, lot tracking, laterality | Drug name, lot, laterality, counseling notes |
Quick tip: Use one comparison handout for all knee injection visits.
When you need a more detailed internal comparison, review Orthovisc Vs Synvisc, Monovisc Vs Synvisc, and Hyalgan Vs Synvisc. For a broad overview that includes other approaches, see Knee Pain Treatment Options.
If your clinic stocks multiple brands, keep product references separate from clinical guidance. Examples listed on the site include Synvisc-One Prefilled Syringe, Orthovisc, and Hyalgan.
Clinic Operations: Procurement, Traceability, and Inventory Controls
A consistent operations playbook reduces errors and wasted inventory. Start with controlled access, then build traceability into everyday steps. Many clinics create an “injectables log” that captures receipt date, storage location, lot number, and the patient encounter where the unit was used. That log supports reconciliation if a manufacturer notice or internal audit occurs.
When procurement teams evaluate vendors, they should confirm licensing requirements, documentation expectations, and how authenticity is verified. Clarify whether products come through a screened distributor network and whether paperwork is available for your compliance file.
One practical way to answer “what is synvisc” for non-clinical staff is: a knee joint viscosupplement that must be tracked like other office-administered injectables. That framing anchors the conversation in handling and documentation, not patient marketing.
- Verify: clinic credentials and authorized users
- Document: product identifiers and receipt records
- Receive: inspect packaging integrity on arrival
- Store: follow the package insert conditions
- Dispense: match laterality and patient record
- Record: lot/expiry in the procedure note
MedWholesaleSupplies focuses on authentic, brand-name medical products for healthcare settings.
If you support multiple sites, align delivery windows with clinic days. Some practices prefer US distribution to reduce transit variability, but policies differ by organization. Whatever your model, keep storage requirements visible in the medication room and avoid mixing look-alike cartons in the same bin.
Authoritative Sources
- AAOS Osteoarthritis of the Knee Clinical Practice Guideline
- FDA AccessData (use for official product labeling lookups)
Further reading: Consider building a one-page staff reference that links your chosen product label, your aftercare handout, and your documentation checklist.
This content is for informational purposes only and is not a substitute for professional medical advice.