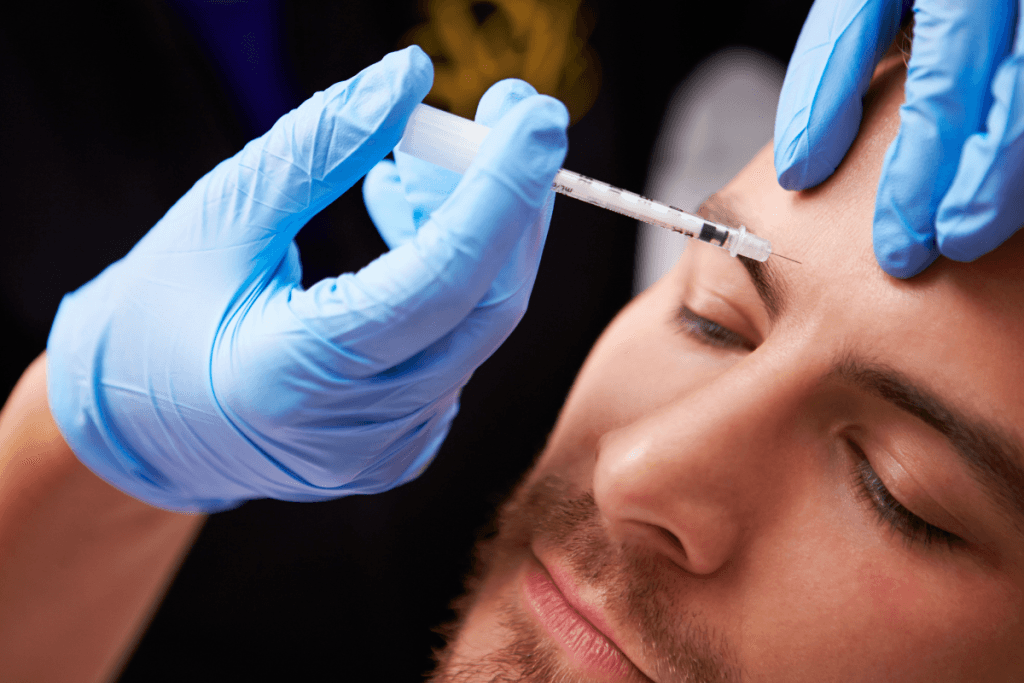
Teams often hear the same patient questions about neuromodulators. One of the most common is: what is Xeomin, and how does it differ from other botulinum toxin type A products. This guide is written for licensed clinics and healthcare professionals who need a practical, operational overview. It focuses on mechanism, typical use cases, how to interpret “before and after” content, and safety signals that show up in real-world conversations. It also covers clinic-facing sourcing and documentation steps that support compliant use.
Key Takeaways
- Mechanism: temporary chemodenervation (reduced muscle signaling) in targeted muscles.
- Use cases: cosmetic lines and certain therapeutic indications, per local labeling.
- Expectations: photos can mislead; standardize imaging and documentation.
- Safety: counsel on local reactions and rare systemic warning signs.
- Operations: verify supply chain, lot data, and storage requirements.
what is Xeomin and Why Clinics Use It
Xeomin is a prescription botulinum toxin type A product used by trained injectors for aesthetic and, in some settings, therapeutic applications. It works by blocking acetylcholine release at the neuromuscular junction, which reduces muscle contraction in the treated area. In plain language, it can relax certain facial expression muscles that contribute to dynamic lines.
Most clinics evaluate it alongside other botulinum toxin products as part of a consistent neuromodulator offering. The operational value is less about “one best product” and more about predictable handling, clear labeling, and fit with your injector training and patient population. For background reading, see Xeomin Purified Overview.
Why it matters: A clear product definition reduces consent confusion and complaint risk.
MedWholesaleSupplies supplies medical products only to licensed clinics and healthcare professionals.
When your practice reviews a product, keep the discussion anchored to the approved label and your internal protocols. Marketing language and social posts tend to flatten nuance. Your job is to translate that into safe, repeatable clinic processes. If your team needs a central place to browse related items, the Xeomin Category Hub can help organize options during formulary review, while keeping clinical decisions separate from procurement.
For clinics that prefer consistent naming across vendors, it also helps to document the nonproprietary ingredient name in your SOPs and EHR templates. That improves chart clarity during cross-coverage and audit preparation. If you maintain a product reference list for staff, linking to the manufacturer’s prescribing information is a simple control.
Indications and Patient-Selection Considerations
In aesthetics, botulinum toxin injections are commonly used to soften the appearance of dynamic facial lines that relate to muscle activity. The most familiar use case is the glabellar complex, but patient requests may also involve the periorbital area (crow’s feet), forehead, and perioral region. Some patient requests, such as changes around the lips, can involve techniques that may be off-label depending on jurisdiction and local labeling. Keep your documentation clear when a request falls outside approved indications.
In therapeutic care, certain botulinum toxin type A products have approvals for specific neuromuscular conditions in some regions. Indications vary by country and by product, so avoid “class-based” assumptions. If your clinicians work across cosmetic and medical lines, confirm that ordering, storage, and administration workflows are aligned with your governance model.
Operationally, patient selection is less about “who is a candidate” and more about whether your clinic can deliver a consistent assessment and follow-up process. That includes standardized photography, medication reconciliation, and a clear adverse event escalation pathway. If your team compares brands across a portfolio, the Botox Category Hub can be a useful way to group similar product types during review meetings, without turning the discussion into brand promotion.
Many clinics also build a short, scripted pre-treatment education handout. It should cover expected onset variability, that results are temporary, and that symmetry outcomes can differ. This reduces the “but I saw it on TikTok” dynamic during consults. For broader context on brand families, Top Botulinum Toxin Brands is a helpful internal primer for new staff.
Interpreting “Before and After” Content in Practice
Patient searches for before-and-after images are common, especially for “eyes” and “lips.” That content can support education, but it can also drive unrealistic expectations. Lighting, camera angle, facial expression, and timing after injection can all change the apparent result. When you review patient-provided photos, treat them as preference signals, not clinical benchmarks.
In consults, it helps to explain what a photo does not show: baseline muscle strength, injection pattern, product handling, and follow-up adjustments. If your clinic publishes images, standardize your own capture process. Use consistent focal length, neutral lighting, and the same facial expression prompts. That makes outcomes easier to audit and reduces dispute risk.
When staff field online comparisons, you can reframe the conversation around controllable variables. A patient asking what is Xeomin “supposed to look like” often needs education on natural variability and how “settled” results differ from early effects. For deeper reading you can share internally, Overview Before And After discusses common themes that appear in outcome galleries.
Clinic photo and notes standards (high-level)
Build a repeatable template that captures what matters for continuity of care. Record the treatment area, lot/expiry information, and a short baseline description of muscle activity. Note any factors that can influence appearance in photos, such as brow position at rest or habitual squinting. Use the same timepoint definitions for follow-ups, and document the patient’s primary concern in their own words. This structure makes later “bad review” investigations faster and more objective.
If your clinic tracks outcomes, consider a simple internal scale for patient satisfaction and functional impact. Keep it consistent across providers. That helps you identify training needs without relying on social media sentiment. For non-clinical trend awareness (not clinical standards), the Beauty Trends Category can help teams understand the language patients use online.
Safety Signals, Adverse Effects, and Counseling Touchpoints
All botulinum toxin products carry important risk information that should guide patient counseling and clinic protocols. Commonly discussed adverse effects include localized injection-site reactions, bruising, and transient discomfort. Headache is also frequently mentioned in post-treatment discussions, although timing and causality can be difficult to interpret. Your documentation should distinguish what the patient reports from what you observe clinically.
More serious risks can involve spread of toxin effect beyond the injection site, which may cause symptoms like generalized muscle weakness, swallowing difficulty, or breathing problems. These events are uncommon, but they are high-impact. Build a clear escalation pathway for front-desk and triage staff so warning symptoms are not minimized or delayed.
Online threads can distort frequency perception. Searches for xeomin side effects reddit or “bad reviews” often cluster around communication gaps: unmanaged expectations, unclear aftercare instructions, and slow responses to symptoms. When patients ask what is Xeomin “doing to my body,” it can help to use plain language: it temporarily reduces signaling at targeted nerve endings, and effects are expected to wear off over time. Refer to the product labeling for full warnings and contraindications.
MedWholesaleSupplies uses vetted distributor sourcing for brand-name medical products.
For long-term concerns, keep the tone balanced. Current medical use involves repeat treatments for many patients, yet long-term safety discussions should stay label-based and individualized. Some patients worry about “toxins building up.” Others worry about changing facial movement over time. You can address these concerns by explaining temporary mechanism, reinforcing follow-up, and documenting patient goals and boundaries.
To support staff education on typical counseling points, How Dysport Works is a useful comparative read, since many questions overlap across products. If you need a reference listing for procurement teams, you can also keep a non-clinical product index such as the Dysport Product Listing in your internal resources.
Comparing Botulinum Toxin Options Without Overpromising
Clinics are often asked to compare products as if they are interchangeable. They are not. Units are product-specific and not directly convertible, and labeling language differs across manufacturers. When you build a comparison, focus on what your clinic can verify: labeled indications in your jurisdiction, storage requirements, reconstitution instructions, and staff competency.
Patient-facing comparisons also tend to exaggerate duration differences. In practice, perceived duration depends on dose selection, muscle activity, and treatment pattern. Keep your messaging conservative. Explain that individual response varies and that follow-up planning matters as much as product choice.
If a clinician asks what is Xeomin “equivalent to,” redirect to the safest framing: it is one option within botulinum toxin type A products, with its own labeling and handling instructions. For deeper internal review, see Xeomin And Botox Comparison and Xeomin Vs Dysport Review.
| Decision factor | What to verify | Why it affects operations |
|---|---|---|
| Label and indications | Jurisdiction-specific prescribing information | Impacts consent language and documentation |
| Units and interchangeability | Unit definitions per product label | Prevents conversion errors across brands |
| Handling and storage | Storage temperature and preparation steps | Drives refrigerator space and workflow timing |
| Training and technique | Injector competency and standard mapping | Supports consistency across providers |
| Pharmacovigilance workflow | AE intake, documentation, escalation steps | Reduces response delays and complaint risk |
Procurement teams also benefit from separating “clinical preference” from “supply reliability.” Build a substitution policy in advance, including how to notify clinicians and how to document product changes. If you keep product references for staff, link them to the correct item pages such as Botox Product Listing and Xeomin Product Listing, while keeping clinical guidance in your SOPs and label library.
Clinic Operations: Procurement, Documentation, and Storage Basics
For neuromodulators, operational discipline prevents most avoidable problems. Start with verification. Confirm that your supplier model supports licensed-professional purchasing and that product pedigree information is available on request. Ensure your receiving staff know how to check tamper evidence, expiration, and lot numbers at intake.
Storage and preparation requirements vary by product and formulation. Do not assume the same handling steps across brands. Train staff to follow the package insert and your internal preparation checklist, and document deviations. If you operate across multiple sites, standardize where products are received, logged, and stored to reduce “missing vial” events.
Quick tip: Keep lot and expiry capture in one place, before the product is shelved.
As a practical response to patient questions, your front desk and coordinators should have a consistent script. When a patient asks what is Xeomin compared with “Botox,” they are often asking about experience and safety, not molecular biology. Your script can explain that it is a prescription neuromodulator administered by trained clinicians, and that product choice is based on labeling, injector experience, and patient goals.
Procurement and documentation checklist
- Supplier verification: confirm licensed-channel fulfillment
- Receiving log: record lot and expiration
- Packaging check: inspect seals and labeling
- Storage SOP: follow labeled requirements only
- Inventory control: track transfers between locations
- Clinical record: document product used and rationale
- AE pathway: define triage and escalation steps
- Training file: maintain injector competency records
Inventory management also affects patient experience. Stock-outs can force last-minute rescheduling, which increases complaint volume and negative online reviews. If your practice depends on US distribution, clarify lead times and documentation expectations with your supplier, and keep a conservative par level.
Common pitfalls that drive “bad reviews”
- Overpromising: implying guaranteed cosmetic outcomes
- Inconsistent photos: changing angles or lighting
- Weak follow-up: slow response to symptom reports
- Unclear aftercare: vague activity guidance
- Chart gaps: missing lot or expiration data
MedWholesaleSupplies focuses on authentic, brand-name medical products for professional clinical use.
When your clinic adds another brand to the shelf, update every downstream step. That includes consent language, staff training notes, EHR pick-lists, and patient education templates. If your team also evaluates related options used in some markets, you can review background pieces like Bocouture Wrinkle Reduction Guide and keep procurement references such as Bocouture Product Listing separate from clinical decision-making.
Finish with a short internal audit every quarter. Sample charts for documentation completeness, match inventory logs to administrations, and review adverse event notes for timeliness. Those steps do more to protect patients and the clinic than any marketing claim.
Authoritative Sources
When you need definitive information, go to primary labeling. It is the best source for indications, contraindications, warnings, and handling instructions. Use it to align your consent documents and staff training materials.
For neutral, authoritative references, start here:
- FDA listing for Xeomin labeling and safety information
- FDA listing for Botox labeling and safety information
Further reading within your team can focus on standardizing consult language, photo protocols, and adverse event workflows. Those operational controls translate well across all botulinum toxin products.
This content is for informational purposes only and is not a substitute for professional medical advice.