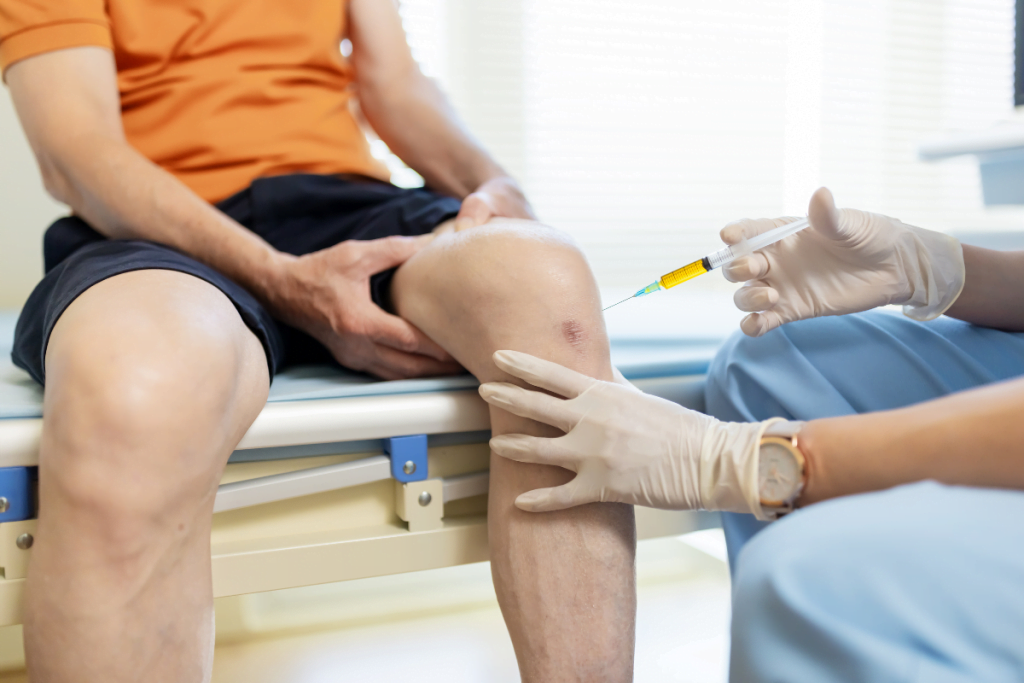
Knee osteoarthritis care often includes injectables that aim to improve joint function. In clinic conversations, rooster comb injections for knees usually refers to intra-articular hyaluronic acid (HA) “gel” injections, also called viscosupplementation (joint-fluid supplementation). The nickname comes from early HA sourcing from rooster comb tissue, though many products are no longer avian-derived.
This guide is written for licensed clinicians and practice teams. It focuses on what the therapy is, what to monitor, and what to document. It also covers common patient questions you will hear during consults.
Key Takeaways
- Clarify product class: HA viscosupplement, not an oral supplement.
- Screen for contraindications and allergy history per labeling.
- Set expectations on onset, duration, and follow-up variability.
- Document diagnosis severity, prior therapies, and payer requirements.
- Prepare for local reactions and post-injection phone triage.
MedWholesaleSupplies supplies only to licensed healthcare professionals and clinics.
Rooster Comb Injections for Knees: Clinical Overview
These injections are typically discussed in the context of symptomatic knee osteoarthritis. The injected material is hyaluronic acid, a naturally occurring component of synovial fluid (joint lubricant). In osteoarthritis, synovial fluid can lose viscosity and elasticity. Viscosupplementation aims to restore some of those mechanical properties within the joint space.
From a workflow perspective, the main operational challenge is alignment. You want the patient’s clinical picture, the specific product selected, and payer documentation to match. Product labeling and payer policies can vary across HA preparations. That variability is also why patients may report mixed “knee gel injection reviews” across different experiences and settings.
Why it matters: Clear definitions reduce rework in prior authorization and follow-up documentation.
Where products fit in a broader injection pathway
In many practices, HA injections sit alongside other office-based options, including corticosteroid intra-articular injections and image-guided procedures. Patients often ask about “rooster comb injections vs cortisone.” You can frame the difference in plain terms: steroids mainly target inflammation, while HA is intended to improve lubrication and mechanical cushioning. Onset and duration are variable for both, and policies differ by payer and plan. Avoid overpromising; focus on shared decision-making and label-aligned counseling.
For deeper background reading, you can reference your internal education pages on How Rooster Comb Injections Work and a general overview of Orthopedic Injection Options.
What They’re Made Of and How This Differs From Supplements
Patients commonly ask what is rooster comb injections made of. In practice, the injectable is a sterile hyaluronic acid preparation intended for intra-articular use. Some products are derived from avian sources, and others are produced through bacterial fermentation. The source can matter for patients with relevant hypersensitivity histories, but the best reference is the individual product label.
It also helps to separate injectables from oral “rooster comb supplement” products. Supplements are not the same dosage form, route, or regulatory category as intra-articular HA. Even when the ingredient name overlaps, the clinical workflow and documentation standards differ. Keep the conversation grounded: you are evaluating a procedure using a labeled medical product, not advising on retail supplements.
Synovial fluid basics in patient-friendly language
Patients may understand HA faster when you relate it to function. Synovial fluid helps reduce friction during movement. Hyaluronic acid contributes to that “slippery” quality. When joint surfaces degenerate, patients can feel grinding, stiffness, and pain with weight-bearing. Viscosupplementation is described as adding a gel-like material to improve the fluid environment. This explanation is not a promise of outcomes. It is a practical way to align expectations and reduce confusion between injections, supplements, and other joint procedures.
Benefits, Limitations, and Selection Signals in Practice
When you discuss pros and cons of rooster comb injections, keep the framing clinical and individualized. Benefits may include symptom improvement for some patients, with a non-opioid, office-based procedure. Limitations include variable response, payer restrictions, and the need for repeat courses in some care plans. The counseling goal is clarity: what the procedure is, what it is not, and how you will assess response.
Patients also ask how long do rooster comb injections last. Duration is not uniform across products or patients. It can depend on osteoarthritis severity, activity level, comorbidities, and the specific HA formulation. Some practices discuss relief in terms of weeks-to-months ranges, but you should anchor any duration language to labeling and your local protocol. Document the baseline functional limits you plan to reassess, such as walking tolerance, stairs, and sleep disruption.
When brand comparisons arise, keep them descriptive rather than promotional. Patients may ask about synvisc injection, Hyalgan, Monovisc, or other viscosupplements. You can point clinicians and staff to comparative reading like Hyalgan Vs Synvisc and Orthovisc Vs Synvisc for structured discussion points.
Safety, Side Effects, and Post-Injection Complaints
Teams should be prepared for questions like what are the side effects of hyaluronic acid injections and knee gel injections side effects. The most common issues discussed in labeling are local reactions, such as injection-site pain, swelling, warmth, and effusion. Less commonly, patients may report marked inflammatory reactions. As with any intra-articular procedure, infection is a serious concern, even if uncommon, and warrants clear escalation pathways.
Patients also report knee pain worse after gel injection, which can prompt after-hours calls. Operationally, it helps to separate expected short-term soreness from red-flag symptoms. Create a consistent triage script that routes urgent symptoms to clinical evaluation. Avoid giving patient-specific medical direction in nonclinical channels. Instead, document the complaint, the timing, and the guidance provided by your licensed staff according to clinic policy.
Common terms you’ll hear at the front desk
Patients may use informal labels like “visco injection knee side effects” when they search online. They may also describe “fullness” in the knee, a limp, or difficulty sleeping after the procedure. Standardize how your team captures these descriptions in the chart. Map them to clinical terms when appropriate (for example, effusion (fluid in the joint), erythema (skin redness), or limited range of motion). Consistent language supports cleaner follow-up, reduces repeat calls, and strengthens payer narratives when coverage depends on documented function.
What to Expect After the Procedure: Activity and Follow-Up
Set expectations early about what to expect after rooster comb injections. Many clinics counsel that mild soreness can occur and that activity recommendations may be modified for a short window. Patients will ask can i walk after gel injection in knee and about gel injection in knee recovery time. Your best answer is process-based: what your clinic typically advises, what the product labeling states, and which symptoms should trigger a call.
Exercise questions are common, including exercise after knee gel injection and what should i do after knee gel injections. Avoid blanket rules in written materials unless they are part of your approved protocol. Instead, align on practical guardrails: avoid unusual overload, follow clinician guidance, and resume activity based on symptoms and professional assessment. Document the instructions provided and who provided them, especially when follow-up is managed by nursing staff.
Quick tip: Put aftercare points in the after-visit summary to reduce call volume.
Clinic Operations: Documentation, Sourcing, and Handling
For practice managers, viscosupplementation can be documentation-heavy. Coverage often depends on the diagnosis, prior conservative therapies, severity, and timing of previous injections. Patients may ask does medicare pay for rooster comb injections. A safer clinic answer is that coverage varies by plan, local policy, and clinical criteria, and that your team verifies benefits before scheduling. Keep benefit verification separate from clinical counseling in the chart, with clear timestamps.
Products are brand-name and supplied with standard documentation on request.
On procurement, use a sourcing process that supports traceability. Many clinics prefer suppliers that support licensed-account onboarding and maintain documented pedigrees where applicable. If you are managing multiple HA product types, standardize receiving steps (lot capture, expiration checks, temperature excursions if applicable per label, and secure storage). If your clinic relies on US distribution for predictable replenishment, document how that aligns with your scheduling cadence rather than promising timelines.
Clinic workflow snapshot (high level)
- Verify patient eligibility and payer rules.
- Document diagnosis and prior therapies.
- Select product per protocol and label.
- Arrange procurement and confirm storage needs.
- Receive, log lot/expiry, and store securely.
- Administer per clinician technique and policy.
- Record product identifiers in the chart.
- Schedule follow-up and capture outcomes.
Documentation and coordination checklist
- Problem list alignment with imaging notes
- Functional limitations stated in plain language
- Prior therapy dates and response summary
- Laterality and target joint confirmation
- Product lot and expiration captured
- Consent and aftercare documented
- Billing codes reviewed for consistency
To connect product selection with operational planning, your team may also reference manufacturer-specific pages such as Synvisc-One Prefilled Syringe or Synvisc Classic Prefilled Syringes for packaging details, while relying on official labeling for clinical use instructions.
Comparing “Gel” Injection Options Without Overpromising
When patients compare types of gel injections for knees, they often assume all products are interchangeable. Clinically, formulations can differ in molecular structure, cross-linking, source material, and dosing schedule format. The most useful comparison points for your team are operational and label-driven: what must be verified, what must be documented, and what follow-up outcomes you will track. In staff training, it can help to use rooster comb injections for knees as a plain-language label, then translate to the exact product name and chart terminology at check-in.
| Comparison point | What to verify in clinic operations | Why it affects workflow |
|---|---|---|
| Source and formulation | Check label for avian vs fermentation and contraindications | Supports allergy screening and consistent counseling |
| Administration schedule format | Confirm whether protocol uses single or multiple visits | Impacts scheduling blocks and follow-up cadence |
| Packaging and identifiers | Capture lot, expiration, NDC/UDI if applicable | Strengthens traceability and billing documentation |
| Payer policy fit | Confirm diagnosis criteria and previous therapy requirements | Reduces denials and rescheduling due to missing proof |
For staff education, internal reading that can help standardize language includes Types Of Gel Injections, Monovisc Knee Injection, and What Is Durolane Injection. If your clinicians are asked about synvisc-one pros and cons, align the discussion to official labeling, patient selection, and your observed tolerability patterns rather than online anecdotes.
Inventory is sourced through distributor partners that undergo supplier vetting.
Authoritative Sources
When updating protocols, use primary sources first. Product labeling is the most reliable reference for indications, contraindications, warnings, and handling requirements. Professional society guidance can help frame when therapies may be considered, but local practice patterns and payer rules still drive operations.
The sources below are useful starting points for clinicians and practice teams who want to confirm definitions and safety framing. They are not a substitute for the product’s full prescribing information and your institution’s policies.
- For osteoarthritis guideline context, see AAOS resources at American Academy of Orthopaedic Surgeons.
- For general osteoarthritis education and patient-facing terminology, see CDC Osteoarthritis Overview.
- For regulatory context on medical products and safety reporting, see U.S. Food and Drug Administration.
If you want deeper internal comparisons, review Orthovisc And Synvisc Comparison and Synvisc Classic Research to support consistent staff explanations.
This content is for informational purposes only and is not a substitute for professional medical advice.