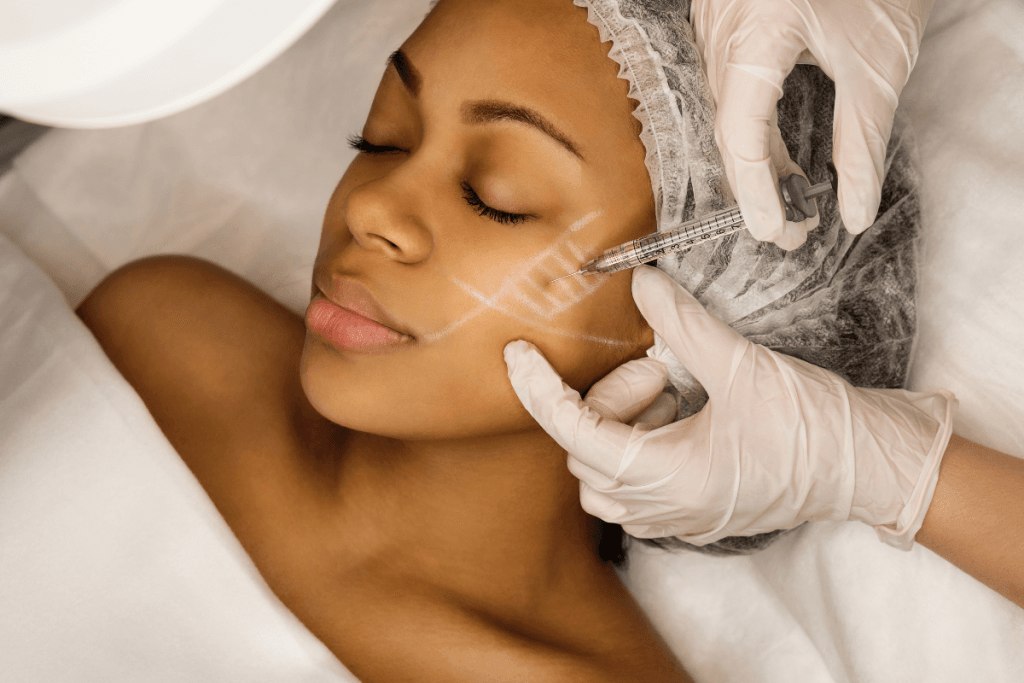
Interest in Profhilo injections has increased as patients ask for hydration-focused injectables. Many people describe goals like “glow,” elasticity, and smoother texture. Clinics also see more screenshot-based expectations from social media. That makes consistent counseling, documentation, and product verification more important than ever.
This guide frames Profhilo as one example within the wider “skin booster” (hydration-oriented injectable) category. It focuses on operational readiness: how to set expectations, handle complaints, and compare adjacent options like HA fillers and biostimulators.
MedWholesaleSupplies supports purchases for licensed healthcare professionals only.
Key Takeaways
- Position skin boosters as texture-focused, not volumizing.
- Standardize photos, timelines, and consent language.
- Plan for common, usually short-lived injection reactions.
- Use clear criteria for when to escalate concerns.
- Verify lot traceability and documentation before use.
Profhilo injections in Practice: What Clinics Should Know
Profhilo is commonly discussed as a hyaluronic acid (HA) injectable used for skin quality goals. In everyday clinic language, it is often grouped with “skin boosters” and “biorevitalizers.” Those labels can confuse patients who expect the lift or contour associated with traditional dermal fillers. Your intake and consent workflow should therefore separate “hydration and texture” goals from “volume and shape” goals.
From an operations view, success depends on alignment more than marketing. You need consistent screening, standardized photo capture, and a predictable follow-up pathway. You also need staff-wide language for what is and is not being promised. That reduces misunderstandings that later show up as profhilo injections reviews that focus on expectations rather than clinical issues.
How It Works at a High Level
Hyaluronic acid is a naturally occurring glycosaminoglycan found in skin and connective tissue. In injectables, HA materials are formulated for different behaviors in tissue. Some are designed to add structure or volume, while others are positioned around hydration and skin quality. Clinics may explain this as “supporting moisture retention” and “improving the look of crepey skin,” without implying guaranteed collagen change or a specific degree of tightening. For staff education, keep mechanisms high-level and avoid product-specific technique claims unless you are referencing official training materials.
How It Differs From Traditional Fillers
A helpful framing is that classic fillers are often selected for contouring or replacing volume in targeted facial compartments. In contrast, skin booster approaches are usually discussed for diffuse surface changes, including fine lines, dullness, and mild laxity. That does not make boosters “stronger” or “weaker.” It makes them different tools for different endpoints. If your practice already uses HA fillers, link the conversation to familiar concepts like viscosity, tissue integration, and expected visibility of product placement, while keeping the discussion neutral and indication-agnostic.
For team alignment across injectable categories, you may want a shared reference hub like the Dermal Fillers Category to keep terminology consistent.
Patient Expectations: Timeline and “Before-and-After” Language
Most dissatisfaction starts with time. Patients may expect dramatic changes after “profhilo after 1 week” because that is how social posts are framed. In practice, early changes—if they occur—are often described as hydration or glow. Later changes, if they develop, may look like more even texture or softer fine lines. Your role is not to promise a timeline, but to define what your clinic will measure and when you will reassess.
Why it matters: Expectations drive complaint volume more than technique details.
Before-and-after standards reduce disputes. Set rules for lighting, camera distance, and facial expression. Use the same background and lens when possible. Document skin prep, makeup status, and recent procedures that can confound interpretation. This is especially relevant when patients search for “profhilo before and after 40s” and bring age-based comparisons that may not match their baseline sun damage or skincare regimen.
Also address the “is profhilo worth it” question in a clinical way. Translate it into decision criteria: the primary concern (texture vs volume), willingness to do a series, tolerance for short-term swelling, and openness to combination planning. When patients cite “is profhilo worth it reddit,” acknowledge the noise in online anecdotes and anchor the discussion in your documented outcomes and follow-up process.
For broader context, the internal primer on Skin Boosters Injections can help teams standardize patient-facing explanations.
When Outcomes Look Wrong: Risks, Complaints, and Triage
Search interest in “profhilo gone wrong” reflects a real operational issue: patients often interpret any post-injection change as a complication. Many effects reported in profhilo bad reviews are common injectable reactions, such as bruising, tenderness, erythema (skin redness), or short-term swelling. These need clear pre- and post-visit instructions and a staff triage script so calls are handled consistently.
At the same time, clinics must remain vigilant for uncommon but serious adverse events that can occur with injectable procedures. Your protocol should define when to escalate internally, when to request photos, and when to direct someone to urgent in-person evaluation. Extra caution is warranted around periorbital (around-the-eye) complaints because “profhilo gone wrong eyes” searches often reflect anxiety about swelling, asymmetry, or visual symptoms. Do not downplay these concerns. Route them through a defined clinical pathway.
MedWholesaleSupplies sources brand-name products through vetted distribution partners.
Common Pitfalls That Create “Gone Wrong” Narratives
- Loose photo standards: inconsistent angles, lighting, or makeup.
- Vague endpoints: “tightening” without defined measures.
- Undocumented add-ons: peels, lasers, or topicals near treatment.
- Delayed callbacks: anxiety grows when follow-up is slow.
- Unclear aftercare: patients resume heat, workouts, or massage early.
If a patient arrives with “profhilo gone wrong pictures,” treat the images as a starting point, not evidence. Ask when each photo was taken, what devices were used, and whether filters were applied. Clarify if the patient is searching “profhilo gone wrong pictures before and after,” which often mixes immediate post-procedure swelling with later baseline photos. Your chart should capture what was reported, what was observed, and what follow-up plan was provided.
For a deeper internal overview of complaint patterns, see Profhilo Gone Wrong.
Comparing Options: Skin Boosters, Fillers, and Biostimulators
Patient shopping often collapses different product classes into one bucket. That shows up in searches like “profhilo vs sculptra” and “profhilo vs fillers.” A practical way to counsel is to compare the primary treatment intent rather than a brand-versus-brand debate. Keep the discussion grounded in what each class is typically chosen to do, the expected follow-up cadence, and how outcomes are assessed.
Combination planning is another frequent topic. Patients may ask about “profhilo and sculptra together” or bring “profhilo vs sculptra vs radiesse” screenshots. Your clinic should decide, in advance, how it sequences different modalities and how it documents informed consent when treatments are layered. Policies vary by practice, training, and local regulations, so keep any public-facing statements conservative.
| Option (category) | Often chosen for | Typical counseling focus | Operational notes |
|---|---|---|---|
| Hydration-focused HA boosters | Diffuse skin quality concerns | Texture, luminosity, fine lines | Photography and timeline alignment matter |
| HA dermal fillers | Contour and volume targets | Shape changes and symmetry | Precise mapping and complication preparedness |
| Biostimulators (e.g., PLLA, CaHA) | Structural support strategies | Gradual change and staged planning | Document sequencing with other procedures |
| Adjunctive “skin quality” injectables | Mixed texture and tone goals | Maintenance mindset and skincare support | Clarify what is on-label vs off-label locally |
If your team wants a structured comparison conversation, the article Jalupro Vs Profhilo can help standardize language around skin-quality injectables. For HA background that supports patient education without overpromising, reference Hyaluronic Acid Overview.
For clinics building a menu, it can help to keep adjacent options in view, such as Viscoderm Hydrobooster Listing and educational summaries like Viscoderm Hydrobooster.
Aftercare, Follow-Up, and Review Management
Aftercare is both clinical and reputational. Patients who search “profhilo aftercare” tend to compare instructions across clinics. When guidance differs, they may assume the procedure is risky or poorly standardized. Build an aftercare handout that is specific enough to reduce confusion, yet general enough to remain accurate across providers and evolving protocols.
Focus on controllables: what normal injection-site reactions can look like, what activities may exacerbate swelling or bruising, and when the clinic wants updates. Avoid rigid promises, especially around longevity, because “profhilo longevity” discussions can be heavily influenced by skin quality, lifestyle, and concurrent treatments. Instead, define a review cadence and what will be assessed at each touchpoint.
Review management also benefits from a process. When you see profhilo injections reviews that mention “nothing happened,” treat that as a signal to improve expectation setting and photographic baselines. When a review describes a negative event, respond with a privacy-first script and offer structured follow-up, without debating details in public. Internally, trend complaints by theme (pain, swelling, asymmetry, perceived lack of change) and update your intake script accordingly.
For counseling language around hydration-oriented injectables, your team may also find Restylane Skinboosters Vital useful as a parallel example of how clinics explain “skin hydration” goals.
Clinic Workflow and Procurement Checklist
Operational readiness matters as much as injector skill. Your clinic should be able to show what was used, where it came from, and how it was stored and tracked. That protects patients, supports incident review, and reduces the “counterfeit concern” conversations that can arise when injectables trend on social platforms.
Quick tip: Build one checklist that covers all injectable skin boosters.
MedWholesaleSupplies is positioned around supplying authenticated, brand-name medical products to verified clinical customers. If you operate with US distribution partners, ensure your internal receiving process still verifies shipments against purchase records, because the responsibility for safe handling and documentation remains with the practice.
- License verification: confirm authorized facility and clinician status.
- Product verification: confirm labeling and tamper evidence.
- Lot tracking: record lot and expiry in the chart.
- Storage check: follow manufacturer temperature and light guidance.
- Informed consent: document goals, alternatives, and expected variability.
- Photo protocol: consistent angles, lighting, and timepoints.
- Adverse event plan: escalation contacts and reporting pathway.
- Waste handling: document disposal per local requirements.
For clinics that want a concrete reference item in their formulary list, link your internal catalog entry to a product record such as Profhilo HL Prefilled Syringe, while keeping patient-facing material non-promotional. If your team monitors broader aesthetic interest for messaging alignment, the Beauty Trends hub can help you anticipate the next wave of questions.
Authoritative Sources
Regulatory status and safety information can vary by country, product presentation, and intended use. When patients ask “is profhilo fda approved,” treat it as a prompt to verify status using primary sources and to explain what “approval” means in your jurisdiction. In the US, FDA resources can help you confirm which dermal fillers are approved and review general safety considerations for injectable soft tissue products.
For neutral, high-quality references, start with these sources and then cross-check with the manufacturer’s official documentation where applicable:
- FDA: Dermal Fillers (overview and safety)
- FDA: Approved Dermal Fillers (product listings)
- American Academy of Dermatology: Dermal fillers
Further reading: If you are building a skin-quality injectable pathway, keep a single internal document that compares classes, sets photo standards, and defines your escalation workflow. That approach is usually more effective than debating individual online anecdotes.
This content is for informational purposes only and is not a substitute for professional medical advice.