MicronJet® Microneedle Device for Clinics
Price range: $11.99 through $318.99
Description
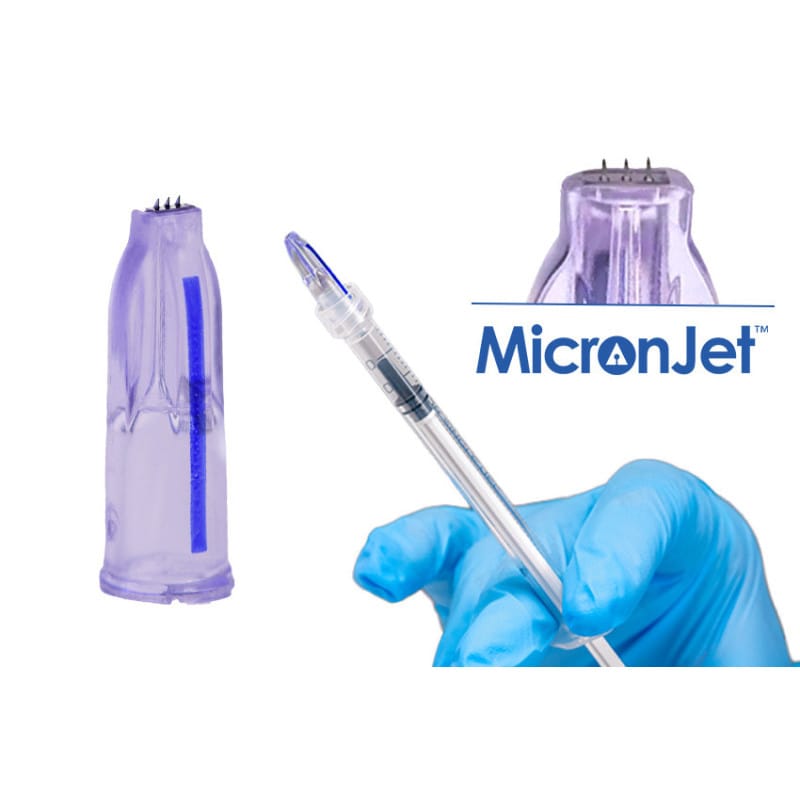
MicronJet® is a sterile micro-needle device engineered for precise intradermal administration in professional care settings. It helps clinicians place small volumes reliably within the dermis while maintaining comfort and procedural control. Clinics stock this platform for consistent placement, reduced tissue trauma, and streamlined appointments, backed by US distribution.
What MicronJet Is and How It Works
This class of micro-needle technology uses very short, fine needles to deliver medications just beneath the skin surface. The array creates a controlled intradermal pathway and forms a uniform wheal, supporting even dispersion without deep penetration. The approach can reduce resistance on injection and helps standardize dermal deposition across sessions and operators.
Med Wholesale Supplies serves licensed clinics and healthcare professionals with authentic, brand-name medical products sourced through vetted distributors. As a MicronJet intradermal device, this platform is designed for accurate, shallow placement that supports intradermal techniques and modern aesthetic workflows. The low-profile needles limit tissue deflection compared with conventional needles, which may aid consistency across treatment areas.
The device is single-use and sterile. It is intended to be attached and disposed according to the manufacturer’s instructions for use. Facilities may incorporate the platform into standard protocols after validating technique on-site and ensuring staff competency for intradermal administration.
Professional Applications
Clinics deploy this intradermal microneedle device across several professional settings. Typical uses include aesthetic skin-quality protocols that call for dermal micro-dosing, regenerative and wellness workflows that require reliable shallow placement, and dermatologic procedures where uniform intradermal delivery is preferred. It can also support training environments by offering a predictable, shallow target plane.
Teams often integrate the platform alongside Mesotherapy programs and device-based rejuvenation. It can fit into pre-existing treatment pathways with minimal change management, allowing staff to standardize dose placement across sessions. For accessory planning and needle alternatives, see Cannulas and Needles or browse our broader Medical Devices category.
Key Features
- Micro-needle array: Creates a consistent intradermal pathway for shallow delivery.
- Controlled depth: Short needles help position fluid just beneath the epidermis.
- Reduced insertion force: Fine geometry supports comfortable skin entry.
- Low tissue disruption: May limit bruising compared with standard needles.
- Sterile microneedle device: Single-use and sterilized to support aseptic technique.
- Workflow friendly: Clear hub design and tactile feedback aid placement control.
- Broad protocol fit: Compatible with many clinic-administered intradermal treatments.
- Quality manufacturing: Medical-grade materials and sealed sterile packaging.
Benefits in Practice
- Consistent dermal placement: Supports repeatable technique across operators and sites.
- Comfort-forward experience: Fine needles can lessen injection sensation for clients.
- Time efficiency: Predictable placement may reduce repeat passes.
- Low learning curve: Familiar handling supports straightforward staff onboarding.
- Inventory control: Single-use microneedle device simplifies sterilization planning.
- Versatile integrations: Fits aesthetic, wellness, and dermatologic care pathways.
Composition & Ingredients
The device is built from medical-grade components suited for clinical use. Needle elements are produced from stainless steel selected for strength and precision. Each unit is assembled and sterilized to support aseptic workflows. Packaging and labelling are designed for traceability across clinic inventory systems.
- Medical-grade stainless steel micro-needles
- Sterile, single-use device components
- Sealed sterile pack with protective cap
Clinics familiar with MicronJet 600 can align their training and technique with that model designation where appropriate. Always consult the product label and instructions for use supplied with your shipment.
Packaging & Supply
Units are supplied sterile and intended for single use. Packaging supports clean transfer to the clinical field and straightforward disposal after use. Labels typically show lot, expiry, and relevant manufacturer details to support your inventory controls and recall readiness.
Available pack counts include 1 or 30 per box, allowing you to align purchases with procedure volume and storage capacity. Facilities that prefer brand-designated variants can reference NanoPass MicronJet 600 when updating protocols or training materials. For related accessories and alternatives, review our Medical Devices category.
Ordering & Logistics
Sign in or create a verified clinic account to view contract and volume tiers. Your facility can consolidate purchases across product lines and request documentation for credentialing or onboarding. Our team can assist with compatibility questions, usage scenarios, and integration into your standard operating procedures.
Once approved, you can place orders aligned to your procedural calendar and receive tracking details through your account portal. For procurement teams standardizing supplies across locations, this catalog entry supports MicronJet for clinic use with clear pack options and reliable sourcing. For best-practice refreshers, see our article on Injection Safety.
Comparable Products
Clinics evaluating alternatives sometimes pair or substitute with micro-needle or micro-cannula devices based on protocol goals and provider preference. Consider Fillmed NanoSoft for delicate intradermal micro-needling in aesthetic workflows. For cases requiring cannula-based placement, see SoftFil Cannula as a flexible, low-trauma option.
Pricing & Access
Pricing is available to verified professional accounts. Sign in to review current tiers, including volume-based and contract pricing structures designed for clinics, med spas, and multi-site providers. Reach out if you require standardized quotes for formulary approval or capital committee review.
Availability & Substitutions
Supply may vary by lot and distribution cycle. If your first-choice configuration is not available, our team can coordinate appropriate substitutions after confirming clinical compatibility with your lead provider. Related cannula options such as Precision Cannula and category resources like Cannulas and Needles can support contingency planning. For purchasing guidance, see Wholesale Cannulas.
Authoritative Sources
Ready to equip your team? Sign in to order this device for your facility with temperature-controlled handling when required and tracked US delivery.
Frequently Asked Questions
How does the device support intradermal technique?
This platform uses very short, fine needles to access the dermis without reaching deeper muscle. The shallow geometry helps form a uniform wheal and supports even dispersion of small volumes. Because insertion forces are low, operators can achieve repeatable placement with controlled depth. The approach also reduces the need for multiple passes, which can streamline session timing and enhance consistency across providers in a busy clinic environment.
What training should staff complete before first use?
Clinicians should be competent in intradermal administration and familiar with the device’s instructions for use. A brief in-service helps align hand position, angle, and volume control across the team. Many facilities run a supervised practice session to confirm consistent wheal formation before live appointments. Document local protocols for skin prep, device disposal, and incident reporting. Always follow institutional policies and manufacturer guidance.
Is it compatible with standard clinic workflows?
Yes. The device is designed to integrate into established workflows with minimal disruption. It supports intradermal dosing used in dermatology, aesthetics, and wellness settings. Inventory handling is simple because units are sterile and single-use. Most teams incorporate it alongside existing injectables after confirming compatibility and updating local checklists for prep, application, and disposal in the clinical environment.
Is the device single-use and how should it be disposed?
Each unit is sterile and intended for single use only. Do not attempt to resterilize or reuse. After the procedure, dispose of the device in accordance with sharps and biohazard policies at your facility. Keep the protective cap and packaging available during setup to support clean handoff and disposal. Inspect units before use and discard if packaging is opened or compromised.
What pack options are available for procurement?
Packs are available as single units or boxes containing 30 devices. This range lets procurement align orders with clinical volume and storage capacity. Labels typically include lot and expiry to support inventory tracking and documentation. If your formulary specifies a particular model designation, reference the label on the shipped unit when reconciling receiving logs and training materials used by your team.
How should the device be stored in the clinic?
Store in the original sterile packaging and protect from moisture, direct sunlight, and temperature extremes. Follow the storage guidance on the product label and manufacturer’s instructions. Do not use if the sterile barrier is compromised or the expiry date has passed. Keep units organized by lot and expiry to support first-in, first-out inventory rotation and efficient periodic checks by your staff.
How does this compare with cannulas or standard needles?
Traditional needles and micro-cannulas can be effective but target different planes. This device focuses on shallow, intradermal placement using very short micro-needles, which can support uniform wheal formation and controlled dispersion. Cannulas may be chosen for subdermal threads or when a blunt approach is preferred. Standard needles are versatile, but can be less consistent for dermal-level micro-dosing compared with a purpose-built intradermal tool.
Specifications
- Main Ingredient:
- Manufacturer: NanoPass Technologies
- Drug Class: Medical Device
- Generic Name: Stainless Steel Micro-needles
- Package Contents: 1 needle | 30 Needles
- Storage Requirements: Room Temperature (2℃~25℃)
- Main Usage:
Here to help
Questions about ordering, delivery or products? You can email our team here or call now at 1-800-630-9757 and be connected with your dedicated Account Manager
Related Products
NEAUVIA™ ORGANIC INTENSE LIPS
RejuvaNAD+
Related Articles
Euflexxa Injection Price: Cost Drivers for Clinics
Key Takeaways Budget drivers: Site-of-care, contracting, and distribution channel matter. Coverage variance: Medicare and commercial…
Durolane Hyaluronic Acid for Knee OA: Clinical Guide
Key Takeaways Product class: A viscosupplement (joint-lubricating injection), not a corticosteroid. Clinical use: Commonly discussed…
Osteoporosis Bone Building Drugs: Anabolic Options for Clinics
Key Takeaways Define the goal: distinguish bone-forming therapies from antiresorptives. Sequence thoughtfully: transitions can matter…
Md Ceuticals Sunscreen Clinical Guide for Clinic Selection
Key Takeaways Md ceuticals sunscreen can be assessed like any clinical sunscreen: verify claims on…
Sculptra Side Effects: What Clinics Should Screen and Track
Key Takeaways Tracking Sculptra side effects starts with clear expectations, tight documentation, and consistent follow-up.…
Hyaluronidase for Lip Filler: Clinical Workflow Essentials
Key Takeaways Hyaluronidase for lip filler is typically used to reverse hyaluronic acid gel in…
What Is Mesotherapy? Clinical Uses, Risks, and Workflow
Key Takeaways Define the technique: what is mesotherapy refers to superficial multi-injection delivery of small…
Juvederm Before And After Photos Documentation Guide For Clinics
Key Takeaways Standardize images: Control pose, lighting, lens, and distance. Document context: Record product, lot,…