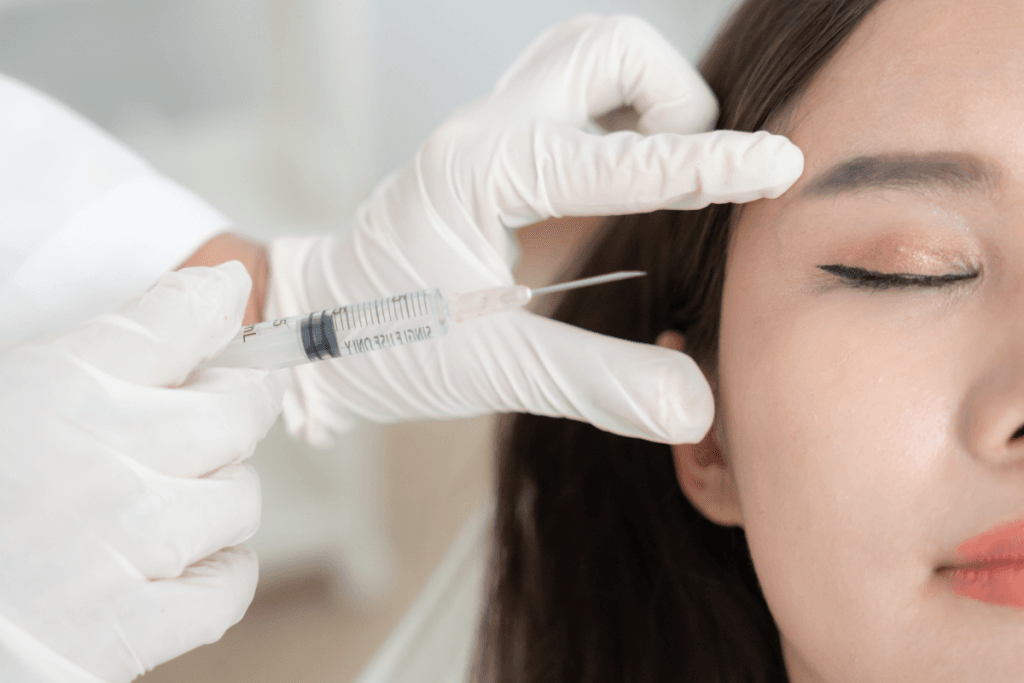
Skin quality injectables keep expanding, and teams need a clear playbook. Plinest injection is often discussed as a polynucleotide-based approach for dermal repair goals. In practice, the key questions are operational. What is it, how does it differ from other “skin boosters,” and what should your clinic document?
This briefing is written for licensed healthcare professionals. It focuses on mechanisms at a high level, realistic outcome framing, and clinic workflows. It does not provide dosing, prescribing, or patient-specific guidance.
Key Takeaways
- Define the category: Polynucleotides aim at skin quality, not volume replacement.
- Standardize evidence: Consistent photos and chart notes reduce “before and after” ambiguity.
- Plan for downtime: Set expectations for transient injection-site reactions.
- Compare thoughtfully: “Versus” claims depend on mechanism, tissue target, and patient goals.
- Operationalize sourcing: Prioritize traceability, storage requirements, and documentation.
Plinest injection: What It Is and Where It Fits
Plinest is generally positioned within polynucleotide therapy (injectables that use DNA fragments as bioactive materials). In everyday clinic language, it is discussed as a “skin quality” injectable. That typically means the intent is to support texture, hydration, and overall dermal appearance, rather than to create projection the way a traditional volumizing filler might.
Clinics usually evaluate fit across three lanes: patient goals, injector skill set, and compliance readiness. Patient goals may include diffuse crepiness, early laxity, or post-inflammatory texture changes. Injector readiness includes comfort with intradermal placement, conservative mapping, and counseling around gradual change. Compliance readiness includes product verification, traceable lot documentation, and clear informed consent language aligned with your local regulations and the manufacturer’s instructions for use (IFU).
In many practices, Plinest is assessed alongside other skin-focused injectables found in Dermal Fillers Category hubs. Some suppliers also list related options or variants intended for different anatomical priorities, such as Plinest and Plinest Eye.
Many suppliers limit distribution to verified licensed healthcare facilities.
How Polynucleotide Skin Boosters Are Thought to Work
Polynucleotides are commonly described as biological polymers that can interact with the extracellular matrix (ECM) and tissue microenvironment. The clinical goal is usually “biostimulation” (supporting the skin’s repair processes) rather than immediate structural filling. Because these products sit within a broad and evolving category, exact composition and handling details can vary by brand and market. Your team should rely on the current IFU and local regulatory status.
Mechanistically, clinics often explain the concept in patient-friendly terms as “supporting skin repair.” In more technical terms, the discussion tends to focus on hydration support, dermal remodeling signals, and improved skin feel over time. This framing also helps when patients compare “plinest vs skin boosters” online. Many boosters share the same appointment format, but they may differ in principal material (polynucleotides vs hyaluronic acid (HA) gels vs hybrid biostimulators) and in the tissue plane emphasized.
Core concepts your team can standardize
To keep staff messaging consistent, define a few internal terms and use them repeatedly. “Skin quality” can mean texture, fine lines, and surface uniformity, not facial contouring. “Biostimulator” can mean an injectable intended to encourage tissue remodeling, with changes that may be subtle and progressive. “Series planning” can mean more than one session may be discussed, but intervals and total sessions depend on the IFU and clinician judgment. For broader orientation to this category, see Skin Boosters Injections and a related PN-focused overview such as Nucleofill Treatment Overview.
Setting Expectations: “Before and After” in a Clinical Context
Patients and staff often look for clear “plinest before and after” examples. The challenge is that skin quality outcomes can be real yet hard to photograph. Lighting, lens choice, and facial expression can change perceived texture more than the intervention itself. Your clinic can reduce confusion by controlling variables and documenting what you are actually trying to change.
Start by translating goals into observable measures. Examples include changes in diffuse dehydration lines, photodamage-associated roughness, or the appearance of post-acne texture. If acne scarring is part of the discussion, keep your language cautious. “Plinest for acne scars” is often searched, but outcomes can vary widely by scar type, depth, and adjunctive procedures. Consider documenting scar morphology (icepick, rolling, boxcar) and whether combination therapy is planned, without promising a specific magnitude of change.
Photography and charting that stand up to review
Standardization is the most cost-effective quality control step you can implement. Use the same camera, distance, and background. Fix lighting position and intensity, and document it in your SOP. Capture at least two angles per zone, and use neutral expression. Record concurrent skincare changes, energy-based procedures, and any recent illness that could affect skin appearance. When staff encounter forum summaries like “plinest vs rejuran reddit,” direct them back to your controlled documentation. Anecdotes can highlight what patients notice, but they are not a substitute for standardized outcomes tracking.
Why it matters: Better documentation reduces disputes about whether change occurred.
Procedure Overview, Injection Points, and Aftercare
Polynucleotide skin boosters are typically delivered via multiple small placements across a treatment zone. The approach is usually about even distribution rather than targeting a single fold. Clinics commonly create a mapping template that the injector can adjust based on skin thickness, vascular risk zones, and the specific concern being treated.
When teams discuss Plinest injection points, keep the conversation at a principles level unless your IFU provides a defined pattern. Many clinics plan by anatomical region (midface, lower face, neck, periocular area) and then refine placement density within each region. Depth and device choice (needle vs cannula) vary by injector preference, training, and patient factors. Maintain aseptic technique, confirm contraindications in your intake workflow, and ensure emergency supplies and escalation procedures are current for any injectable service line.
Aftercare and downtime planning
Set expectations for short-term visible effects that come from the injection itself. Common post-procedure experiences across many intradermal injectables include transient erythema (redness), edema (swelling), tenderness, and bruising. Your aftercare handout should describe what is typical, what is less common, and what triggers a same-day call. Downtime can also be social rather than medical, so align scheduling guidance with the patient’s work and events calendar.
Operationally, treat aftercare as part of risk management. Provide written instructions, document that they were given, and note any deviations from standard post-procedure plans. If you reference other clinic educational materials for HA-based boosters or mesotherapy-style cocktails, link staff to consistent internal reading. As external background, your team can review Restylane Skinboosters Vital Overview and Fillmed NCTF 135 HA Guide to understand how adjacent categories are described and documented.
Safety, Side Effects, and Contraindications
Every injectable program needs a uniform safety script. With Plinest injection, many concerns overlap with other intradermal treatments because the needle entry and tissue response drive much of the early risk. Build your consent and counseling around injection-site reactions, infection prevention, and the uncertainty that comes with individual variability. Avoid overstating expected results or minimizing adverse outcomes.
Commonly discussed adverse events after skin boosters can include short-lived swelling, bruising, localized nodules, or prolonged inflammation. Less common but higher-consequence risks exist with any facial injectable, including vascular compromise and serious infection. Your clinic should have clear escalation pathways, staff role assignments, and documentation templates for adverse event follow-up. Contraindications and precautions vary by product and jurisdiction, but often include active infection at the site, certain hypersensitivity histories, and situations where elective procedures should be deferred. Confirm the current IFU and align with your medical director’s protocols.
Quick tip: Keep an adverse event log with lot numbers and photos.
For brand-name injectables, suppliers should be able to support authenticity documentation.
Comparing Options: Rejuran, Profhilo, Juvelook, Nucleofill
Patients will ask “plinest vs rejuran” and similar matchups because brand names are easy to repeat. A better internal framework is mechanism first, then tissue target, then workflow impact. In broad terms, polynucleotide products are discussed as repair-oriented. HA-based boosters are often framed around hydration and fine lines. Hybrid biostimulators may emphasize longer remodeling arcs, with different counseling needs.
Plinest injection comparisons also need market context. Product composition, labeling, and indications can differ by country, and that changes what you can claim and how you train staff. If your team wants side-by-side reading, start with neutral clinical summaries like Rejuran Skin Booster Guide and Viscoderm Hydrobooster Overview, then map what overlaps and what does not.
How to compare (clinic-facing decision factors)
- Primary material: Polynucleotide vs HA vs hybrid biostimulator.
- Target plane: Superficial dermis vs subdermis emphasis.
- Outcome framing: Texture shift vs hydration feel vs structural change.
- Visit planning: Series language, follow-up cadence, documentation needs.
- Tolerability profile: Expected downtime and patient acceptance.
| Category | Typical clinic framing | Documentation focus | Common workflow implications |
|---|---|---|---|
| Polynucleotide injectables | Dermal repair and skin quality support | Texture, tone, and scar appearance notes | Emphasize gradual change and standardized photos |
| HA skin boosters | Hydration and fine-line softening | Dehydration lines and patient-reported feel | Downtime counseling often centers on bruising |
| Hybrid biostimulators | Remodeling-oriented “collagen support” messaging | Longer interval follow-up comparisons | More emphasis on longer-term tracking consistency |
To keep sourcing conversations grounded, separate educational reading from procurement. If your formulary includes specific branded items, ensure staff use correct naming and avoid cross-brand claims. Where relevant to your service line, product listings like Rejuran Healer or Nucleofill Eyes can be referenced for catalog alignment, while clinical counseling should remain anchored to approved labeling and your protocols.
Clinic Operations: Sourcing, Documentation, and Workflow
Operational readiness often determines whether a new injectable is successful in your clinic. Start with governance. Define who approves formulary additions, who maintains IFUs and consent language, and who owns adverse event reporting. Then build traceability steps into receiving and stocking so you do not rely on memory later.
When Plinest injection is added to a menu, align three documents on day one: your intake contraindications checklist, your procedure note template, and your aftercare sheet. This reduces variation between injectors and makes outcomes easier to audit. It also supports consistent language when patients ask about “plinest vs profhilo” or “plinest vs juvelook,” where expectations can drift if staff improvise.
Procurement and receiving checklist (non-exhaustive)
- License verification: Confirm facility credentials match supplier requirements.
- Product identity: Verify labeling, lot, and expiry on arrival.
- Chain-of-custody: Record receiving date and receiving staff initials.
- Storage SOP: File IFU handling requirements in a shared location.
- Traceability: Link lot numbers to patient records consistently.
- Returns plan: Document your procedure for discrepancies or damage.
- Verify licensure and authorized users in your system.
- Document IFU, contraindications, and consent language updates.
- Receive inventory, record lot/expiry, and store per IFU.
- Dispense/administer per protocol and chart lot traceability.
- Track outcomes with standardized photos and follow-up notes.
Reputable suppliers typically source through vetted distributors to support traceability.
For staff training, keep a short reading list that explains adjacent categories without turning into marketing. The internal articles linked earlier can support that baseline: Skin Boosters Injections, Nucleofill Treatment Overview, and Rejuran Skin Booster Guide.
Authoritative Sources
Because product regulation varies by country, use authoritative sources to anchor your safety and consent language. Start with regulator guidance on injectable adverse events and general filler safety, then layer in your product’s current IFU and internal protocols. When staff encounter social media summaries, redirect them to these references and to your clinic’s own standardized documentation. This approach supports consistent counseling and defensible recordkeeping.
For general safety frameworks and injection practice standards, consider these references:
- FDA Dermal Fillers
- CDC Injection Safety
- PubMed (search polynucleotide injectable reviews relevant to your market)
Further reading should support your internal training and audit process. Focus on consistent terminology, traceability, and patient communication standards.
This content is for informational purposes only and is not a substitute for professional medical advice.