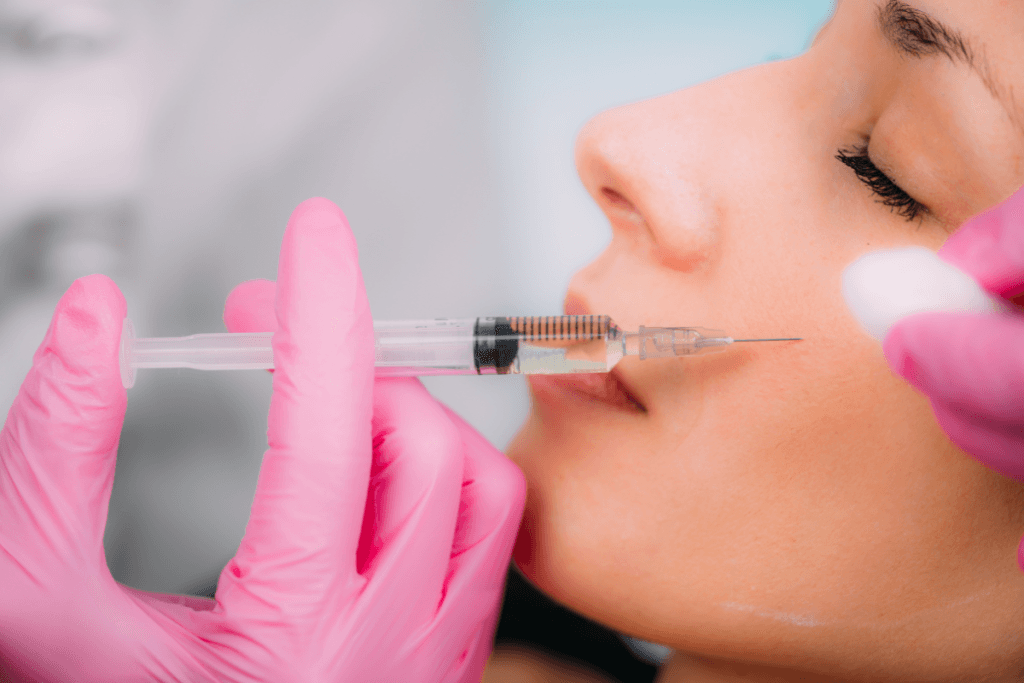
Clinics often evaluate biostimulatory injectables when patients want lift plus skin quality changes. Calcium hydroxylapatite filler sits in that middle ground. It can add immediate volume while also supporting collagen remodeling over time. For practice teams, the real work is selection, counseling, and documentation. Your outcomes depend as much on technique and communication as on product choice.
Key Takeaways
- Think in “immediate support” plus “collagen response,” not one or the other.
- Compare CaHA with HA gels and PLLA based on reversibility, tissue behavior, and follow-up cadence.
- Set expectations for early swelling, bruising, and photo variability.
- Build a workflow for verification, lot capture, and adverse-event reporting.
Where This Biostimulatory Filler Fits in Practice
Calcium hydroxylapatite (CaHA) is a mineral-like material related to calcium and phosphate found in bone. In aesthetic injectables, it is typically formulated as microspheres suspended in a gel carrier. That structure is why it behaves differently than hyaluronic acid (HA) gels. Many teams also discuss it as both a filler and a “collagen stimulator,” because tissue response can evolve after the initial placement.
For clinics building a dermal injectable menu, it helps to organize options by function. HA products are often chosen when reversibility and soft contouring are priorities. PLLA (poly-L-lactic acid) products are often framed as gradual volumization through collagen formation. CaHA is often considered when you want structural support and potential skin quality improvement in one treatment plan. If your team needs a broad refresher on categories, the site’s Types Of Dermal Fillers overview and the Dermal Fillers browsing hub can help standardize internal language.
Why it matters: Clear product framing reduces misaligned expectations and preventable follow-up calls.
Trust cue: MedWholesaleSupplies works with licensed healthcare practices and clinical purchasers.
Calcium hydroxylapatite filler: Mechanism and Tissue Response
At a high level, CaHA products are designed to provide an initial scaffold and then integrate with surrounding tissue. The carrier gel contributes to early contour change, while the microspheres can support a longer-term tissue response. Many clinicians describe this as “biostimulation” (a process that encourages collagen production). Patients may hear “collagen booster,” but your counseling should stay concrete and conservative.
Immediate contour vs longer-term change
Teams often get pulled into the debate behind searches like “is radiesse a filler or biostimulator” and “is radiesse filler.” In operational terms, you can treat this as a continuum. The early visual change is typically driven by placement and the gel’s space-occupying effect. Subsequent change, when it occurs, reflects remodeling in the treated area and is less predictable from a single photo. That is why “before-and-after” assets should be framed as examples, not promises, and should be tied to standardized photography and lighting.
When you document your consult, consider capturing what the patient is actually pursuing: projection, contour definition, skin texture, or a mix. This helps you map the product choice to a goal and reduces later disputes. For staff training, it can also help to define the calcium hydroxyapatite formula in simple terms: mineral microspheres in a gel carrier, intended to provide support and stimulate tissue response.
How to Compare CaHA With HA and PLLA (Sculptra-Type)
Most practices compare CaHA against two main alternatives: HA fillers and PLLA stimulators. Each class has strengths and trade-offs that matter for risk management, scheduling, and patient satisfaction. When patients ask about “radiesse vs sculptra,” they are often comparing treatment timelines and the feel of results. When they ask “radiesse vs filler,” they may mean CaHA versus HA gels, even if they do not use those terms.
Rather than rely on “top 10 filler brands” lists, set up a repeatable comparison. Focus on reversibility options, how the product integrates, the kind of edema you see early, and what follow-up touchpoints you typically require. A simple decision aid can keep consults consistent across injectors.
| Decision factor | HA gel fillers | CaHA class | PLLA class |
|---|---|---|---|
| Primary planning focus | Shape and hydration | Support + potential collagen response | Gradual volumization plan |
| Reversibility planning | Often discussed due to hyaluronidase use | Less straightforward; plan conservatively | Not typically reversible; plan conservatively |
| Photo expectation setting | Often earlier “settled” look | May evolve with remodeling | Often staged changes over visits |
| Operational impact | More SKU variety by area | Technique training on dilution concepts | More emphasis on multi-visit protocols |
If you want a deeper product-class comparison for volume restoration planning, see Comparing Calcium Hydroxylapatite And Poly-L-Lactic Acid Filler and the planning-oriented Sculptra Vs Filler Comparison Guide.
Calcium hydroxylapatite filler vs hyaluronic acid comparisons should also include clinic constraints. HA products may be preferred when you anticipate frequent mid-course adjustments. CaHA may be chosen when you want firmer support and accept that later changes may be less “undoable.” Your local regulations, label, and injector experience should guide that decision.
Technique Concepts, Dilution, and Interpreting Photos
Online searches can push patients toward very specific expectations. Common examples include “diluted radiesse before and after” and “hyperdilute radiesse technique.” In clinic terms, dilution discussions are really about how the product is deployed to emphasize skin quality versus projection. Different approaches may change palpability, spread, and early edema. Because protocols vary widely by training and jurisdiction, it is safest to treat these as technique frameworks rather than fixed recipes.
What patients mean by “swelling pictures”
Patients will sometimes bring “radiesse swelling pictures” and ask if their outcome is normal. Your team can reduce anxiety by explaining the limits of online photos. Swelling depends on depth, vascularity, cannula versus needle approach, compressive forces, and individual bruising tendency. It also depends on camera angle and lighting. Consider building a standardized internal set of counseling points that separates early expected effects (tenderness, edema, bruising) from signs that warrant prompt clinical review per your protocol.
Photos can also create confusion in off-face areas. Searches like “radiesse before and after buttocks” circulate widely, but they rarely communicate what was done, by whom, or under what regulatory framework. If your clinic does not treat an area, say so clearly. If you do offer off-label treatments, keep consent, documentation, and complication readiness at the center of the discussion. When relevant, direct staff to vetted education sources and ensure your photo consent language is unambiguous.
For teams comparing CaHA to an HA volumizer, the Radiesse Vs Juvederm Voluma article can help structure patient-facing explanations without oversimplifying.
Safety Profile: Expected Effects and Escalation Planning
As with any injectable, adverse outcomes can range from mild and transient to serious. Common short-term effects discussed in clinical settings include swelling, bruising, erythema, tenderness, and temporary asymmetry. Patients also ask about “radiesse side effects” and “radiesse side effects long-term.” Your response should stay grounded: risks depend on technique, anatomy, sterility, and patient-specific factors, and long-term concerns are best discussed in the context of labeled safety information and post-procedure monitoring.
Build a practice standard for how you document events, even when they are mild. Consistent notes help you spot patterns across injectors and support quality improvement. They also simplify reporting if escalation is needed. Align your adverse-event process with the manufacturer’s guidance and local requirements, and train front-desk staff on how to route urgent calls. Calcium hydroxylapatite filler side effects long term is a search phrase, but in clinic operations the more useful frame is “what do we track over time and how do we respond.”
Quick tip: Use a single, clinic-approved aftercare handout for all injectables.
Trust cue: Inventory is sourced through vetted distribution partners, supporting traceability for clinical documentation.
Patient Selection, Contraindications, and Aftercare Systems
Patient selection is less about “who wants volume” and more about anatomy, skin quality, and risk tolerance. Your screening should capture bleeding risk factors, prior procedures, history of hypersensitivity, and any active infection at the planned site. You should also document prior filler type where possible, since mixing materials across planes can complicate future assessments. This is where a structured intake helps, even in busy aesthetic schedules.
When staff search “calcium hydroxylapatite contraindications,” they are usually looking for a practical checklist. Keep your language aligned with the product’s labeling and your medical director’s protocol. In general terms, contraindications and precautions often relate to hypersensitivity, infection/inflammation at the injection site, and situations where risks outweigh benefits. Avoid turning these into blanket rules. Instead, make sure your consult and consent flow captures the relevant history and that you have a plan for deferring treatment when needed.
Aftercare processes should be consistent across your injectables, with add-ons when appropriate. Calcium hydroxylapatite filler aftercare conversations often focus on what is normal in the first days and what should trigger a call. Your team should not diagnose by phone, but you can set clear boundaries for when you want in-person assessment. If you treat multiple service lines, also clarify that calcium hydroxyapatite vocal cord injection is an otolaryngology procedure in a different setting with different products and protocols. That distinction prevents unsafe assumptions based on name similarity.
Clinic Workflow Snapshot: Verification, Storage, and Recordkeeping
Even excellent injection technique can be undermined by weak procurement controls. Create a lightweight, repeatable workflow that supports authenticity, traceability, and clean documentation. This matters during audits and when investigating unexpected reactions. It also helps when patients return months later and cannot remember what they received.
Below is a generic process map you can adapt to your policy environment:
- Verify credentials and facility purchasing authority.
- Document product name, lot, and expiration on receipt.
- Confirm packaging integrity before stocking.
- Store per labeling and clinic SOPs.
- Dispense/administer per medical director protocols.
- Record treated areas, device used, and patient counseling.
- Track adverse events and follow-up outcomes.
Receiving checklist for injectable inventory
Use a quick receiving checklist that your clinical and purchasing teams both recognize. It should include lot capture, expiration checks, and a defined quarantine step if anything looks inconsistent. If you are building your formulary, consider grouping SKUs by “types of filler brands” and use-cases, rather than by marketing names. For category browsing, teams often start with Dermal Fillers Collections. For product-specific references during inventory planning, you may see clinics cross-check examples like Radiesse 1.5 mL With Lidocaine, Sculptra 2 Vials, or Lanluma V to align naming conventions in the EMR.
- Match invoice to shipment details
- Capture lot and expiration
- Inspect seals and labeling
- Log any temperature concerns
- Quarantine questionable units
- File documentation in one place
Operationally, Calcium hydroxylapatite filler requires the same disciplined traceability as any implantable or injectable device. If you support multiple locations, consider a single template for lot logging across sites. If your supplier ships via US distribution, document receiving conditions consistently for every delivery.
Trust cue: Clinics can request documentation to support brand-name product authenticity and chain-of-supply review.
Authoritative Sources
Keep your internal protocols aligned with current regulatory and specialty guidance. Product labeling and regulator safety communications should be the baseline for contraindications, handling, and adverse-event reporting. When patients cite social media, these sources help your team reset the conversation to verifiable information.
For neutral background reading, start with these references and then consult the specific product’s official labeling:
Further reading: For a practical discussion of collagen-focused positioning and counseling language, see How Radiesse Boosts Collagen and Sculptra Aesthetic Stimulating Collagen. If your clinic tracks emerging patient expectations, the Beauty Trends hub can be a useful pulse-check, alongside your own outcomes data.
This content is for informational purposes only and is not a substitute for professional medical advice.