Injectable lipolysis (fat breakdown) sits between skincare and surgery. It can help address localized adipose tissue (fat) deposits. It is not a substitute for obesity management. For clinics, the operational work matters as much as technique.
This guide focuses on aqualyx treatment as one example of fat-dissolving injections. It summarizes where it may fit, what “before and after” evidence can and cannot show, and how to reduce avoidable risk. It also covers clinic-facing controls, like documentation, sourcing, and competency tracking.
If you are building or auditing a contouring service, use this as a framework. Confirm local regulatory status, scope-of-practice rules, and manufacturer instructions. Policies vary by jurisdiction and facility type.
Key Takeaways
- Define the service: Local contouring, not general weight loss.
- Standardize records: Mapping, consent, photos, aftercare, and incident logs.
- Plan for reactions: Swelling and bruising are common operational issues.
- Evaluate evidence carefully: Social reviews and photos are often low-quality.
- Control supply chain: Verify provenance and lot documentation consistently.
Where aqualyx treatment Fits in Body Contouring
Clinics usually consider injectable adipocytolysis (fat-cell disruption) when patients want small-area contour changes without surgery. These services often overlap with other aesthetic offerings, so clinical governance needs to be explicit. Define what your clinic will treat, who is eligible, and when to refer out.
In practice, demand clusters around “stubborn fat” areas and photo-driven expectations. This makes consultation structure critical. You will want consistent language for what is realistic, what is uncertain, and what depends on follow-up and technique. A helpful starting point is an internal overview that matches your protocols to the evidence base and local rules. For background, see Aqualyx Clinical Overview.
MedWholesaleSupplies serves verified licensed clinics and healthcare professionals only.
Operationally, be clear that localized contouring is different from medical weight management. Some practices offer both, but they involve different screening, monitoring, and documentation burdens. If your team also supports obesity pharmacotherapy, keep pathways separate and role-defined. You can browse related hubs like Weight Loss Category and review service-alignment considerations in Weight Loss Injections.
Product selection should be handled like any other clinical supply decision. When you evaluate an item such as Aqualyx 10 x 8 mL Vials, treat it as part of a documented protocol, not a marketing feature. Ensure your team can support ordering controls, inventory tracking, and adverse-event escalation.
Planning the Procedure: Training, Mapping, and Technique Limits
Most “how to inject aqualyx” searches reflect a training gap, not a need for shortcuts. Injectable contouring requires anatomy knowledge, aseptic technique, and complication readiness. Your risk profile increases when staff rely on informal videos or “aqualyx training manual pdf” files shared without provenance.
Training and competency documentation
Start by defining who performs which steps and under what supervision. For many clinics, a safe baseline includes role-specific training records, annual competency checks, and a clear escalation chain. Your documentation should capture training source, date, and scope, plus any device- or product-specific modules. If a manufacturer or authorized trainer provides materials, store the current version and retire older copies. Avoid distributing uncontrolled PDFs across personal devices.
Build training around operational scenarios, not just injection technique. Include consent language, photo standards, sterile prep, waste handling, and how to respond to unexpected reactions. If your team needs a structured overview of pre-visit setup and patient instructions, use Aqualyx Injections Procedure as a checklist template, then adapt it to your governance.
Quick tip: Keep one controlled protocol version in your QMS, not multiple “latest” copies.
Treatment mapping, injection points, and record structure
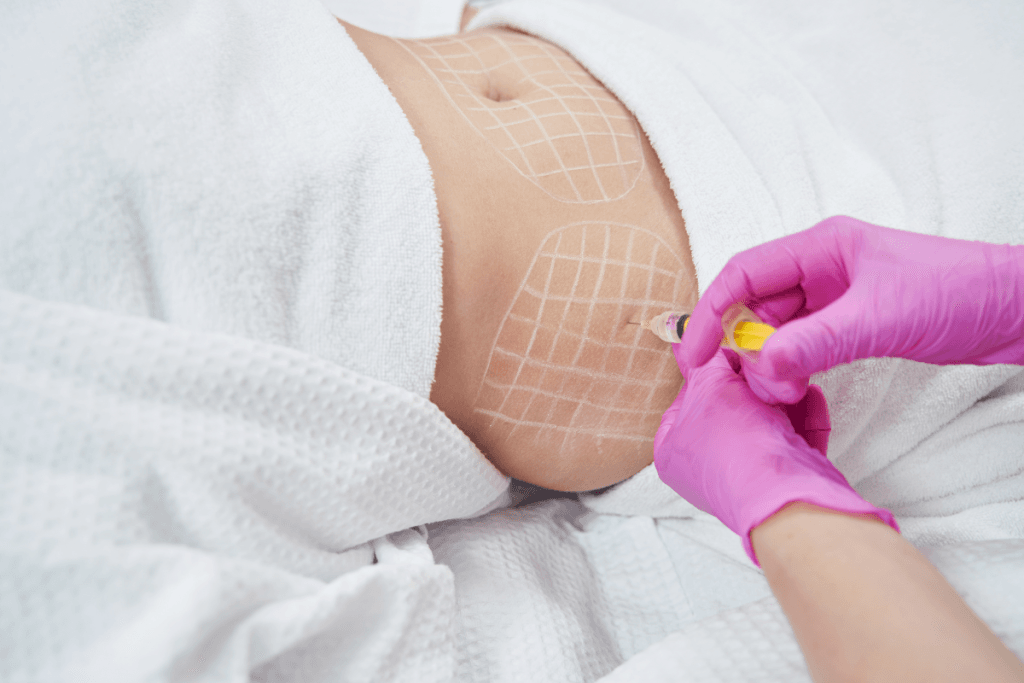
From an operations view, “aqualyx injection points” is a documentation challenge as much as a clinical one. Your notes should show the treated area map, planned entry sites, and what was actually performed. This is especially important for abdomen and submental areas, where “before and after tummy” or “before and after face” expectations are high. Use standardized body diagrams and photo angles to reduce ambiguity when different staff review the chart.
Many practices also see questions like “how much aqualyx to inject per area” or “how many vials of aqualyx for stomach.” Do not answer these with a generic number in policy. Volumes and spacing depend on anatomy, product guidance, and clinician technique. Instead, document that dosing and delivery parameters follow the current manufacturer instructions and training standards, with deviations justified in the record.
If your clinic offers alternative lipolytic approaches, treat them as separate protocols. Ingredients and handling may differ. For example, some teams compare phosphatidylcholine-based approaches for contouring support. For background reading, see Phosphatidylcholine Benefits and maintain separate workflow notes for products such as Phosphatidylcholine 5 Vials.
Managing Safety: Side Effects, Swelling, and Red Flags
Patient counseling often centers on bruising and tenderness, but your clinic should plan for a wider range of issues. “Aqualyx swelling” is a common operational driver of after-hours calls, photo messages, and unscheduled visits. Set expectations for common local reactions, and document what you advised and when.
When teams look up “aqualyx side effects,” they may be trying to triage what is expected versus what is concerning. Keep your communication neutral and systematic. Describe local inflammatory reactions in plain language. If you use clinical terms like panniculitis (inflammation of subcutaneous fat), define them once for staff scripts. Ensure staff know what is within protocol monitoring and what requires escalation to a clinician.
Why it matters: Most “gone wrong” stories involve delayed recognition and poor documentation.
Online searches for “aqualyx gone wrong” or “aqualyx gone wrong stomach” often blend mild, expected reactions with rare complications. Clinics should avoid dismissive language and instead use a consistent triage pathway. Your incident log should capture onset, severity, photos if appropriate, contact attempts, and the final disposition. This supports patient safety and helps you identify training gaps.
Long-horizon concerns also appear in practice. Terms like “aqualyx side effects long term” and “aqualyx side effects liver” come up when patients worry about systemic effects. Be cautious and evidence-based. Emphasize that data quality varies by product and jurisdiction, and that systemic risk assessment should follow your medical history process. If a patient has complex comorbidities, align with your medical director’s policy on whether the service is appropriate in your setting.
- Common pitfall: Inconsistent photo angles across visits.
- Common pitfall: No written triage script for swelling calls.
- Common pitfall: Mixing protocols between different injectables.
- Common pitfall: Poor lot and expiry capture in charts.
- Common pitfall: Relying on unofficial training documents.
Evidence Signals: Before-and-After Images and Reviews
Teams frequently field “aqualyx before and after” questions, but most public images are not clinical-grade evidence. Lighting, posture, camera distance, and timing can change the appearance of contour dramatically. Even “aqualyx before and after 1 treatment” posts can be misleading if they capture peak swelling or a different body angle.
To evaluate outcomes internally, standardize your own photography and measurements. Use the same background, lens distance, and patient stance. Record dates and any relevant confounders, such as recent illness, hydration changes, or concurrent treatments. When the record is consistent, your clinicians can distinguish true contour change from variance and edema.
MedWholesaleSupplies focuses on authentic, brand-name medical products for licensed professional use.
Patient-reported “aqualyx reviews” can still be useful, but treat them as qualitative. Reviews overrepresent extremes, and “aqualyx reviews reddit” threads often lack clinical context. Build a clinic review process that separates service issues (scheduling, follow-up) from clinical concerns (unexpected reactions). Use that feedback to improve scripts, consent clarity, and aftercare materials.
Be careful with “aqualyx gone wrong photos” in consults. Some images are mislabeled, edited, or unrelated to the product in question. If you use photographs for education, ensure you have consent, clear provenance, and appropriate clinical framing. For broader context on how clinics position localized injections within non-surgical contouring, see Fat Dissolving Injections and keep marketing claims aligned with your documentation standards.
Comparing Options: Injectable Lipolysis and Alternatives
Clinics often need to answer “aqualyx vs lemon bottle” type comparisons. Approach this as a decision about workflow fit, training requirements, and risk controls, not as a promise of superior results. In many settings, the best choice is the one your team can deliver consistently, document reliably, and support with follow-up.
It also helps to separate localized contouring from systemic weight management. GLP-1 therapies, for example, address metabolic disease and weight reduction pathways, not spot reduction. If your practice offers both service lines, protect clinical quality by using separate eligibility screens and separate outcomes tracking. Educational context is available in Ozempic For Weight Loss.
| Approach | Typical Clinic Use | Operational Considerations |
|---|---|---|
| Injectable lipolysis (example product) | Small-area contour support | Training, mapping, photo standards, swelling triage |
| Alternative injectable solutions | Protocol-dependent contour services | Do not mix techniques; separate consent and aftercare |
| Device-based non-invasive contouring | Area reduction without injections | Device maintenance, staff credentialing, consistent imaging |
| Surgical options | Larger-volume body contouring | Referral pathways, perioperative risk management |
| Obesity pharmacotherapy | Medical weight management | Screening, monitoring, medication documentation |
If you support multiple injectable options, keep comparisons balanced and documented. For example, some teams maintain a separate protocol for Lemonbottle Ampoule Solution and align patient education with a published overview such as Lemon Bottle Fat Dissolving. Where applicable, you may also benchmark against other injectable approaches described in Alidya Vs Aqualyx, while keeping in mind that product claims and regulatory status can differ by region.
Finally, clarify permanence expectations. Patients often ask “is aqualyx permanent,” but permanence depends on many factors and should never be framed as guaranteed. Keep language conservative. Focus on what your clinic can control: protocol consistency, documentation quality, and appropriate follow-up.
Clinic Operations: Documentation, Sourcing, and Inventory
Your clinical outcome is tied to operational reliability. That includes how you verify products, how you store them per instructions, and how you document each administration. Aqualyx treatment should sit inside the same governance system you use for other prescription and procedure supplies.
MedWholesaleSupplies sources products through vetted distributor channels.
Below is a high-level workflow snapshot you can adapt to your QMS. Keep it short and auditable. Then add local details for your licensing, scope-of-practice, and record retention rules.
Clinic workflow snapshot (high level)
- Verify clinician credentials and scope
- Document consultation, consent, and photos
- Confirm product provenance and lot details
- Receive stock and record expiry dates
- Store per manufacturer instructions
- Perform procedure and chart treated areas
- Provide written aftercare and contact pathway
- Record follow-up and any adverse events
For supply chain controls, use a two-step check: procurement verifies the vendor and documentation; the clinical lead verifies product labeling and protocol match. This reduces mix-ups across look-alike injectables and helps when staff rotate. If you operate with US distribution, ensure your receiving logs capture chain-of-custody and lot traceability.
Use a short internal checklist for each shipment and each clinic day:
- Receiving: Lot, expiry, and package integrity recorded
- Protocol match: Correct product for the scheduled service
- Storage: Location and conditions meet instructions
- Charting: Mapping and photo set completed
- Follow-up: Contact route and escalation documented
Keep product links separate from protocol documents. Staff should know where to find both. For example, procurement may reference Aqualyx 10 x 8 mL Vials for item identification, while clinicians reference your controlled clinical pathway. This separation reduces the risk of informal “protocol drift.”
Authoritative Sources
For injection safety principles and clinic compliance baselines, start with high-level guidance from major public health and regulatory bodies. These sources will not replace manufacturer instructions or local rules, but they help frame your governance.
For internal training and patient education materials, keep your references consistent and current. You can also use these site resources for staff alignment: Procedure Preparation And Aftercare and Fat Dissolving Injections Overview. Update your documents when products, regulations, or staffing models change.
This content is for informational purposes only and is not a substitute for professional medical advice.