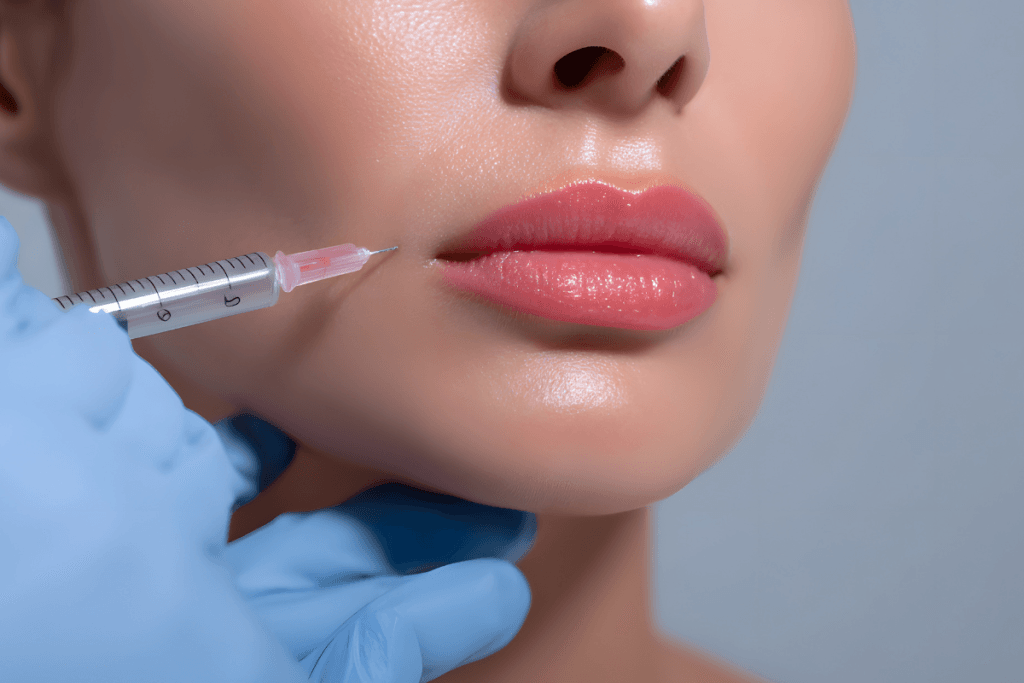
Clinic teams often get pulled into “which is better” debates. In sculptra vs filler comparisons, the useful question is operational: which modality fits the indication, follow-up cadence, and risk controls you can support. That means looking beyond marketing terms and focusing on material class, expected onset, reversibility, and how you document outcomes.
This guide frames poly-L-lactic acid (PLLA) collagen stimulators, hyaluronic acid (HA) gels, and other common injectables in practical planning terms. It is written for licensed clinicians, practice managers, and procurement teams who need a defensible, repeatable selection process.
For background browsing, many teams start with a broad hub like Dermal Fillers before narrowing to product class and anatomy.
Key Takeaways
- Class first: Match material class to clinical goal and reversibility needs.
- Timeline matters: Build plans around expected onset and follow-up capacity.
- Anatomy is not uniform: Risk profile changes by plane and vascular territory.
- Standardize documentation: Photos, lot tracking, and consent reduce avoidable disputes.
Sculptra vs filler: What Changes in Treatment Planning
The phrase “filler” is used loosely in everyday clinic conversation. Operationally, it helps to separate space-occupying gels from biostimulators. A biostimulator (collagen-stimulator) aims to trigger the body’s own collagen response over time. By contrast, many HA products primarily provide immediate physical volume and contour through space-occupying gel properties.
That distinction changes how you plan visits, how you set expectations, and what you measure. With collagen stimulators, you may need a longer documentation window to evaluate change. With HA gels, you often evaluate shape, symmetry, and integration sooner. Your team also needs a consistent language for patient-facing education, especially when patients reference “before and after” images or anecdotal threads from social platforms.
MedWholesaleSupplies supports verified licensed clinical accounts for professional use.
For deeper reading on collagen stimulation concepts, see Sculptra Aesthetic Stimulating Collagen.
Material Class and Mechanism: Volume, Structure, Biostimulation
In day-to-day workflow, classification reduces confusion. HA fillers are gels that can add volume and hydration-like effects, often with a more immediate contour change. Calcium hydroxylapatite (CaHA) products are commonly described as providing structural support and may also have a biostimulatory component, depending on placement and technique. PLLA products are typically framed as collagen stimulators, with visible change emerging gradually.
When clinicians or patients say they want “natural results,” they may mean different things: less immediate swelling, a softer contour, or a slower change that attracts less attention. The best planning conversations translate those preferences into measurable endpoints, such as contour, volume restoration, or skin quality. If your clinic uses multiple classes, keep class-level education materials updated and consistent with each product’s labeling in your jurisdiction.
When teams document a sculptra vs filler choice, a simple rationale often holds up well: immediate contour needs, reversibility requirements, and the practice’s ability to monitor delayed-onset issues.
| Modality (Class) | High-level mechanism | Planning notes | Common operational flags |
|---|---|---|---|
| Hyaluronic acid (HA) gels | Space-occupying gel; water-binding properties | Often selected for contour adjustments and shape refinement | Reversal readiness, adherence to safe injection techniques, vascular risk training |
| Poly-L-lactic acid (PLLA) | Biostimulation (collagen-stimulator) rather than instant volume | Set expectations for gradual change and staged evaluation | Delayed nodules risk counseling; longitudinal photo protocols |
| Calcium hydroxylapatite (CaHA) | Structural support; may stimulate collagen over time | Consider structural goals and tissue characteristics | Technique standardization; management pathway for firmness |
If your team wants a class-based refresher, Types Of Dermal Fillers and Hyaluronic Acid vs Non Hyaluronic Acid Fillers provide structured overviews you can align with your internal protocols.
Anatomic Planning: Matching Product Behavior to Facial Zones
Anatomy drives both outcomes and risk. The same product class can behave differently in thin skin, mobile areas, or regions with complex vasculature. Planning by zone helps your clinic avoid “one product solves everything” thinking, which is a common driver of rework and dissatisfaction.
In sculptra vs filler selection for specific areas, many teams separate “shape and projection” goals from “global volume restoration” goals. They also define what counts as success. For example, a midface plan might prioritize support and contour, while lower face plans may focus on shadow reduction and smooth transitions. Whatever your clinic’s style, write it down. This makes provider coverage, training, and post-treatment documentation more consistent.
Midface, Cheeks, and Temples
Midface volume loss can present as flattening, shadowing, and changes in overall facial proportion. Cheek planning usually involves assessing support, skin thickness, and the patient’s tolerance for a visible immediate change versus gradual change. Temples have variable skin thickness and anatomy, so many clinics treat them as a separate risk and consent pathway rather than “just more volume.”
Because patients frequently bring comparison images, define how your clinic interprets “before and after” sets. Standardize lighting, angle, expression, and time between images. This reduces misinterpretation of swelling, weight change, or differences in head position.
Lower Face: Jawline, Folds, and Marionettes
Lower-face requests often bundle different concerns: jowling, jawline definition, smile lines, marionette shadows, and generalized laxity. These can require different approaches, and sometimes non-injectable modalities. A clinic-level planning model should clarify what injectables can reasonably address and what should be triaged to another consultation pathway.
Operationally, lower-face work benefits from strong documentation: baseline asymmetry notes, patient priorities, and a plan for staged refinement if needed. If the clinic carries multiple HA options, a category hub like Hyaluronic Acid Dermal Fillers can help procurement keep class-level inventory organized without overcommitting to a single SKU.
Some practices also see questions about hands or body areas. These uses may be jurisdiction- and product-specific, and off-label considerations can apply. Keep your policies aligned to local regulations, product labeling, and your medical director’s standards.
Products are sourced through distributor partners that undergo vetting.
Timeline, Longevity, and Maintenance: Setting an Operationally Realistic Plan
Patients often interpret “how long it lasts” as a guarantee. Clinics should treat longevity as a variable influenced by product class, injection plane, metabolism, and treatment goals. From a workflow perspective, what matters is whether results are expected to appear immediately, gradually, or in stages. That affects follow-up scheduling, photo timing, and how you handle “nothing happened” check-ins.
When you document sculptra vs filler planning, consider defining your evaluation windows and communication cadence in advance. For example, you might set separate internal checkpoints for early contour review versus later tissue quality review. This reduces reactive scheduling and helps you route concerns to the right clinical staff member.
Why it matters: Follow-up capacity often determines which injectable plan is sustainable.
Maintenance planning also benefits from clarity on reversibility and adjustment. Many HA products are considered reversible with hyaluronidase when clinically appropriate, while collagen stimulators are typically approached with different management pathways. Avoid promising timelines. Instead, use structured language such as “often gradual” or “may require staged sessions,” and reference the product’s prescribing information for specifics.
For teams comparing biostimulatory classes, Calcium Hydroxylapatite and Poly L Lactic Acid can be a useful framework for staff education.
Safety Profile, Adverse Events, and Patient Communication
All injectable fillers carry risk. Your clinic’s safety posture should be visible in training, consent language, emergency readiness, and documentation. While specific adverse event profiles vary by product and patient factors, common categories include bruising, edema, infection, inflammatory reactions, nodules, and vascular compromise. Vascular occlusion is a rare but serious event associated with dermal filler injections and requires immediate recognition and escalation per your protocol.
In sculptra vs filler counseling, clinics often need two scripts: one for immediate post-injection expectations and another for delayed-onset concerns. Patients may report firmness, asymmetry, or “lumps” after the initial swelling window. Your staff should know how to triage these reports, what information to collect, and when the clinician needs to assess in person. Avoid over-reassurance in written messages. Keep communications neutral and documented.
Quick tip: Document baseline anatomy and standardized photos before any injectable plan.
- Missed documentation: No lot number or injection map recorded
- Unclear expectations: “Natural” not defined in measurable terms
- Inconsistent photos: Lighting and angles vary between visits
- Weak triage: Delayed concerns routed without clinician review
- Stock substitution: Last-minute product switches without updated consent
Many patient questions reference specific brands and comparisons (for example, Juvederm, Restylane, or CaHA options). Keep these discussions anchored to class behavior and training. If your clinic stocks multiple lines, consider maintaining a short internal comparison sheet that includes: class, intended use (per label), reversibility considerations, and the clinic’s preferred documentation fields.
Procurement and Documentation Workflow for Injectable Modalities
A consistent workflow helps clinical outcomes and compliance. It also reduces waste and last-minute substitutions that can undermine patient trust. For procurement teams, class-level planning is as important as SKU-level planning. It helps you keep a balanced mix across HA gels, collagen stimulators, and structural agents, without overstocking highly specific items.
When documenting sculptra vs filler decisions, try to capture the minimum viable dataset: clinical goal, anatomy, product class, and follow-up plan. Then add traceability fields that support audit needs. Many clinics also track which education handout was provided, since patient understanding is a frequent driver of complaints.
Clinic Workflow Snapshot (High Level)
- Verify licensed account and authorized users
- Document product selection rationale and consent
- Receive inventory and record lot/expiry
- Store per manufacturer requirements
- Administer per clinician training and protocol
- Record injection map and post-care instructions
- Log adverse events and report per policy
- Traceability: Lot, expiry, and patient chart linkage
- Authentication: Source verification and documentation retention
- Segregation: Separate training stock from clinical stock
- Substitution control: Update consent if product changes
- Returns policy: Clarify handling requirements with supplier
- Incident pathway: Define escalation and reporting steps
Inventory examples vary by clinic preference and training. Some teams standardize around a collagen stimulator option like Sculptra 2 Vials or comparable PLLA products such as Lanluma V. Others maintain a structured HA lineup and add a structural agent like Radiesse 3 mL when appropriate for their protocols.
If your clinic needs brand examples for HA gels used in contour work, products such as Restylane 1 mL With Lidocaine and Juvederm Volux With Lidocaine are commonly referenced by trained injectors. Selection should remain aligned to labeling, training, and internal governance.
Inventory typically focuses on authentic, brand-name products with traceable distribution documentation.
For procurement browsing, some practices prefer a consolidated hub like Dermal Fillers Product Category. If your clinic depends on reliable US logistics, document receiving checks and temperature exceptions consistently.
Authoritative Sources
- FDA overview of dermal fillers and safety considerations
- FDA consumer update on dermal filler risks
- Manufacturer prescribing information for Sculptra Aesthetic
Further reading: If you are building a training deck, focus on class-based differences, documentation standards, and your clinic’s escalation pathway for complications. Keep brand comparisons secondary to the protocols you can execute consistently.
This content is for informational purposes only and is not a substitute for professional medical advice.
________________________________________________________________________________________
Medically Reviewed by: Ma Lalaine Cheng.,MD.,MPH