Osteoporosis care often becomes urgent after a fragility fracture or rapid bone loss. In that setting, teams may evaluate anabolic (bone-forming) options alongside antiresorptives (bone-loss blockers). Evenity injection is one such therapy, and it raises practical clinic questions about mechanism, safety screening, administration workflow, and how to plan next steps after a time-limited course.
This guide focuses on clinic operations and high-level clinical concepts. It is not a prescribing resource. Always confirm decisions against the current prescribing information and your local protocols.
Catalog access is intended for licensed clinics and healthcare professionals.
Key Takeaways
- Mechanism: A sclerostin inhibitor with dual bone effects.
- Safety focus: Cardiovascular warnings require careful screening workflows.
- Administration: Subcutaneous injection handling and site comfort matter.
- Stopping: Plan for follow-on therapy to maintain gains.
- Operations: Tight documentation supports audit and traceability needs.
Evenity injection: Mechanism and Clinical Fit
Romosozumab (brand name Evenity) is a monoclonal antibody that targets sclerostin. Sclerostin is a signaling protein that normally limits bone formation. By inhibiting it, romosozumab can increase bone formation and also decrease bone resorption. That “dual effect” is the main reason it is discussed differently than classic antiresorptives, such as bisphosphonates or RANKL inhibitors.
Clinically, it is typically considered for patients with osteoporosis at high fracture risk, as defined in labeling and guidelines. In practice, “high risk” is operationalized through prior fracture history, very low bone mineral density (BMD), and other risk markers. Your clinic may also need to align criteria with payer policies, specialty pharmacy rules, and infusion/injection capacity.
Evenity action in plain terms
Staff education is easier when you translate mechanism into shared language. You can describe romosozumab as a bone-building therapy that also slows breakdown. That framing helps patients understand why the course is not “forever,” and why follow-on therapy is often discussed early. It also helps your team separate it from bisphosphonates (which bind bone mineral and reduce resorption) and from denosumab (a RANKL inhibitor that reduces osteoclast activity). Keep the explanation consistent across prescribers, nurses, and prior authorization notes to reduce rework.
Quick tip: Standardize one mechanism paragraph for your prior-auth templates and visit summaries.
Administration Logistics and Injection-Site Considerations
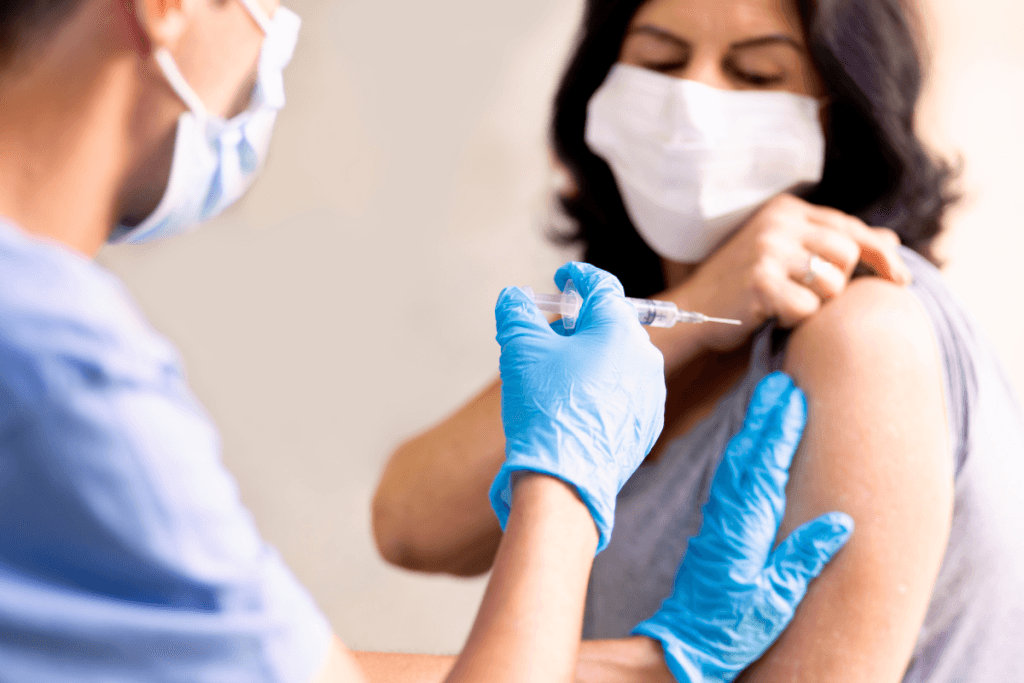
Administration planning starts with the basics: where the injection is given, who administers it, and what competencies are required. Romosozumab is administered subcutaneously, so many outpatient clinics can integrate it into an injection visit model. Even when a therapy is “just a shot,” operational details still drive patient experience and documentation quality.
When patients ask about Evenity injection sites, most clinics use standard subcutaneous locations such as abdomen, thigh, or upper arm, while following the product label and institutional policy. Site selection also intersects with staff ergonomics, patient mobility limits, anticoagulant use, and whether the visit includes teaching. Build a consistent approach to site rotation to reduce localized irritation and to improve record clarity.
Preparing for day-of administration
Day-of workflows often succeed or fail on small steps. Confirm product identity, expiration, and storage conditions per manufacturer requirements before the patient arrives. Verify baseline labs or clinical prerequisites required by your protocol, and document the review in a standardized location in the chart. Many teams also pre-brief patients on common injection reactions and expected follow-up touchpoints. This reduces after-hours calls and keeps adverse event documentation more consistent.
Items are supplied as authentic, brand-name medical products.
Patients sometimes report evenity injection site pain or redness. That is common with many subcutaneous therapies. Your nursing documentation should capture site used, reaction description, onset timing, and any immediate interventions per protocol. Consistent language makes later chart review and pharmacovigilance reporting more reliable.
Safety, Warnings, and Monitoring Conversations
Safety review is not a single checkbox. It is a sequence: screening before starting, monitoring during therapy, and clear escalation guidance if symptoms occur. Staff should be familiar with the boxed warning and contraindications described in the prescribing information. Build these points into both the prescribing workflow and the injection visit checklist.
For Evenity injection side effects, clinics most often prepare for injection-site reactions and musculoskeletal symptoms, while also taking cardiovascular risk seriously. The product labeling includes a warning about risk of myocardial infarction, stroke, and cardiovascular death. Your intake and follow-up forms should support consistent capture of cardiovascular history, recent events, and current symptoms, with clear routing rules for clinician review.
Why it matters: A missed cardiovascular red flag can become a high-risk adverse event and a documentation gap.
Patients may also ask, “does evenity cause cancer?” Current labeling is the best anchor for these discussions. Avoid speculation and avoid overstating reassurance. A practical response is to explain that the known risks and warnings are described in the official prescribing information, and that clinicians monitor for adverse events and report suspected reactions through standard channels.
For a deeper operational summary, see Evenity Side Effects Common And Serious Risks. Keep your internal materials aligned with those core points, but always defer to the label for definitive wording.
Stopping Therapy and Transition Planning
Patients and payers frequently ask what happens when you stop evenity. From a workflow standpoint, the key is to treat “stop” as a planned milestone, not an unplanned interruption. Build reminders early so the team can coordinate follow-on therapy discussions, repeat imaging timing, and coverage checks without last-minute escalations.
How long does evenity stay in your system is another common question. As a monoclonal antibody, romosozumab is cleared through biologic pathways rather than renal excretion, and drug exposure generally tapers over weeks to months after the last dose. Exact persistence varies by patient factors and is best discussed using the pharmacokinetic information in prescribing materials. In clinic documentation, focus on the last administration date, the planned next-step therapy, and any adverse events observed near discontinuation.
From a patient-communication standpoint, be explicit about what follow-up looks like. Some teams schedule a transition visit near the end of the course. Others rely on an osteoporosis care pathway that automatically prompts next steps. Either approach works better when responsibilities are assigned clearly across prescriber, nursing, and billing teams.
Comparing Options for Osteoporosis Services
Clinics often evaluate evenity vs prolia in the context of fracture risk, patient comorbidities, and operational fit. The comparison is not only clinical. It also touches staffing, visit frequency, monitoring processes, and payer rules. When you frame it this way, your team can reduce friction between clinical intent and real-world delivery.
Evenity injection may be considered when a bone-building approach is appropriate and when cardiovascular risk screening is feasible within your intake workflow. Denosumab (Prolia) is an antiresorptive option and often fits long-term maintenance pathways, but it also carries its own discontinuation considerations that require planning. Avoid “either/or” thinking. Many real-world pathways involve sequencing therapies over time, based on guidelines and individual risk.
| Decision factor | Romosozumab (Evenity) | Denosumab (Prolia) |
|---|---|---|
| Therapy type | Bone-building with antiresorptive effect | Antiresorptive (RANKL inhibitor) |
| Course planning | Time-limited course, then transition planning | Often ongoing therapy with structured follow-up |
| Key safety workflow | Cardiovascular screening and symptom escalation | Calcium status and discontinuation planning |
| Clinic operations | Injection visit + documentation consistency | Injection scheduling + continuity of care |
For extended reading, you can cross-reference Evenity vs Prolia Which Is Better For Bone Health and Prolia Injection Effective Solution For Long-Term Bone Health. Use these to support staff education, not as a substitute for labeling.
Procurement, Documentation, and Workflow Checklist
Operational readiness determines whether therapy starts smoothly. Teams often underestimate how much time is spent on benefits verification, prior authorization, and documentation cleanup. You can reduce delays by pre-building a single pathway that covers clinical notes, billing data, and product traceability.
Sourcing routes use vetted distribution channels.
In a wholesale model, clinics may prefer predictable inventory practices and reliable US logistics, while staying within facility policy. Keep procurement decisions aligned with your compliance requirements and payer rules. If your organization uses buy-and-bill, clarify how product receipt, storage, and charge capture are reconciled. If a specialty pharmacy ships to clinic, define who checks integrity, logs receipt, and documents lot details.
For teams browsing related therapy areas, a practical hub is Treating Osteoporosis. If you maintain internal catalog references, you may also map therapy entries to specific listings such as Evenity Non-English, Prolia English Alternative, and Prolia Non-English 60mg 1 1ml Prefilled Syringe. Use listing names only as internal references, and confirm NDCs, packaging, and storage details against the manufacturer documentation you rely on.
Clinic workflow snapshot
A simple workflow snapshot helps new staff and float nurses. Start with verification, then move through receipt and documentation, and end with follow-up planning. Keep it high-level so it can fit different sites. Most process gaps occur at handoffs, such as between prior authorization and scheduling, or between product receipt and charge capture. Audit those handoffs first.
- Verify: eligibility, coverage pathway, and documentation requirements.
- Document: indication basis, risk screening, and baseline assessments.
- Receive: confirm product identity and storage conditions.
- Store: follow manufacturer requirements and site policy.
- Administer: record site, date, and any immediate reactions.
- Record: lot/expiry where required by policy.
- Plan: schedule next visit and transition milestone.
One more practical note: if patients bring outside stories or evenity patient reviews, treat them as context, not evidence. Acknowledge the experience, then pivot to your clinic’s standardized counseling and safety monitoring. This maintains trust without letting anecdote drive care decisions.
Authoritative Sources
- FDA prescribing information for romosozumab
- Bone Health & Osteoporosis Foundation
- Mayo Clinic drug overview: romosozumab
This content is for informational purposes only and is not a substitute for professional medical advice.