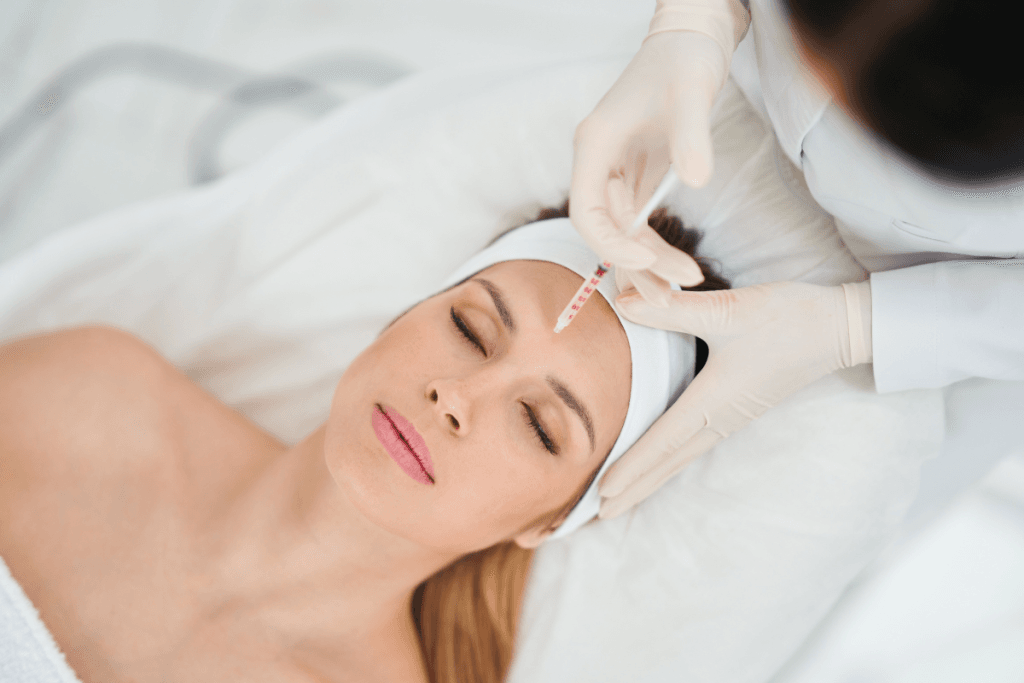
Neurotoxin treatments sit at the intersection of clinical technique and patient expectations. Dysport injections are one option within botulinum toxin type A products. For clinics, the main questions are practical. What is it doing biologically, what outcomes are reasonable to discuss, and what safety signals deserve escalation?
This guide frames Dysport at a high level for licensed healthcare teams. It focuses on mechanism, common use cases, expected timing, adverse-effect triage, and documentation-aware operations. It avoids dosing and patient-specific medical advice.
Why it matters: Clear counseling and consistent workflow reduce avoidable follow-up and dissatisfaction.
Key Takeaways
- Botulinum toxin products have non-interchangeable units and distinct labeling.
- Most adverse effects are local and time-limited, but rare spread effects matter.
- Standardized aftercare messaging prevents common “what did I do wrong?” calls.
- Before-and-after communication should emphasize variability and consented photography.
- Procurement steps should document authenticity and controlled clinical distribution.
Dysport injections: Mechanism, Uses, and Expected Timing
Dysport (abobotulinumtoxinA) is a botulinum toxin type A product. Like others in its class, it works by blocking acetylcholine release at the neuromuscular junction. The result is reduced signaling to targeted muscles, which can soften expression-related lines when used for aesthetic indications and reduce overactivity in certain therapeutic contexts.
Indications and approved sites vary by country and by label updates. Common labeled uses in many regions include treatment of glabellar lines (frown lines) and selected neuromuscular conditions such as cervical dystonia (a neck muscle spasm disorder). Some practices also discuss off-label applications; if you do, keep the conversation anchored to local regulations, training, and informed consent standards.
When patients ask “how fast will I see it,” avoid a single timeline. Onset and peak effect can vary with anatomy, technique, and individual response. Setting a range and emphasizing follow-up assessment is usually more defensible than promising a specific day of change. For deeper background, your team may also reference the clinic-focused overview in Dysport In Depth Look.
Access to neurotoxins is typically limited to verified licensed healthcare accounts.
Safety Signals Clinics Should Track and Document
Most patient-reported effects after Dysport injections are local and mild. You will still want a structured triage approach, because a small subset of symptoms can indicate a higher-risk scenario. Documentation quality matters here as much as clinical assessment.
Common local reactions and “normal” variability
Typical short-term issues include injection-site pain, swelling, bruising, redness, and headache. Some patients report a tight or heavy sensation as muscle activity changes. With forehead treatments, clinics commonly field concerns about asymmetry, brow heaviness, or eyelid droop (ptosis). These are not unique to one brand and can be influenced by anatomy, placement, and post-procedure behavior.
Patients also compare experiences online. Dysport side effects reviews and anecdotal threads can amplify rare outcomes and understate selection bias. When a patient cites social posts, treat it as a cue to clarify symptom details and timing, not as a clinical data source.
Rare but important events to escalate
All botulinum toxin products carry warnings about toxin effect spread beyond the injection site. While uncommon, symptoms such as difficulty swallowing, speaking, or breathing warrant urgent evaluation. Train staff to identify red flags, document onset, and follow your escalation pathway.
Be prepared to address allergy concerns in plain language. Signs of allergic reaction to Dysport can include hives, swelling of the face or throat, wheezing, or severe dizziness. These scenarios require emergency-level evaluation rather than routine follow-up messaging.
Some patients report dysport side effects anxiety, especially when they perceive asymmetry early. Anxiety itself is not a specific pharmacologic signature. Still, it can coincide with shortness of breath complaints that need careful screening. Use a calm script, confirm objective symptoms, and escalate when respiratory or neurologic red flags appear.
For additional operational framing, you can direct staff to Understanding Side Effects as internal reading.
Aftercare Messaging That Reduces Unplanned Follow-Ups
Aftercare is often where clinics win or lose trust. Patients frequently interpret normal variability as a complication. Your goal is consistent, non-alarming guidance that aligns with the product labeling and your medical director’s protocol. Dysport injections after care discussions should be standardized across clinicians, front desk, and nursing staff.
Common questions cluster around sleep, activity, and social events. Dysport aftercare sleeping concerns often involve fear of “moving product” by pressure. Many practices advise avoiding direct pressure or rubbing on treated areas for a defined period and sleeping in a way that does not compress the injection region. Exact instructions vary by clinic protocol, indication, and injector preference.
Activity guidance is another recurring theme. Dysport after care exercise and dysport aftercare alcohol questions come up because patients are trying to avoid bruising or swelling. Rather than giving rigid promises, offer clear “avoid for now” categories that your clinic can defend and apply consistently.
Quick tip: Put your most common aftercare answers into one approved handout.
A practical solution is a clinic-branded dysport aftercare instructions pdf. It can include: expected sensations, what not to do immediately, who to call, and what symptoms are urgent. Keep it short enough to read. Also log that you provided it, along with the lot number and product identifier used.
- Keep instructions consistent across staff and shifts.
- Separate expected effects from warning symptoms.
- Document the counseling and the handout version.
- Use plain-language synonyms for medical terms.
- Define your follow-up contact pathway clearly.
Products sourced through documented, vetted distribution channels support cleaner traceability when questions arise.
Comparing Options: Dysport, Botox, and Other Toxins
Most clinics eventually need a structured way to discuss dysport vs botox and related options. Patients also arrive with screenshots of dysport vs botox reddit threads. Your internal framework should be calmer and more technical than online comparisons.
Start with what is universally true and label-aligned. Botulinum toxin products are not interchangeable. Dysport vs botox units are product-specific, and conversion shortcuts are a common cause of documentation and expectation problems. Keep your training, protocols, and consent language product-specific.
When comparing formulation families, it helps to avoid overclaiming. Different products may vary in manufacturing, accessory proteins, storage requirements, and diffusion characteristics discussed in the literature. The clinical significance of these differences can depend on indication, injection strategy, and patient anatomy. For deeper internal reading, see Botox vs Dysport Analysis and Xeomin vs Dysport Comparison.
| Decision factor | What to standardize in your clinic |
|---|---|
| Label and indication | Use indication-specific consent and charting templates. |
| Units and reconstitution | Keep product-specific training; never assume unit equivalence. |
| Patient counseling | Use consistent timelines and uncertainty language across brands. |
| Storage and handling | Follow manufacturer instructions; log refrigerator temps per policy. |
| Switching products | Document rationale, prior response, and lot numbers carefully. |
If you maintain a formulary view, browsing a hub like Botox Category can help teams see which toxin families are stocked for clinical use.
Setting Expectations: “Before and After” Communication Without Overpromising
Patients routinely search dysport injections before and after content before booking. They also ask for dysport injections before and after pictures in consults. Those assets can educate, but only if captured and presented responsibly. Your policies should address consent, image ownership, and how edits are prohibited.
Operationally, define a consistent photography setup. Use the same lighting, background, camera distance, and facial expression prompts. If the patient changes makeup, angle, or brow position, apparent “results” become hard to interpret. Add documentation notes about baseline asymmetry, prior toxin exposure, and recent procedures that can confound appearance.
When patients ask where to get Dysport on face, they may mean “which areas are treated.” Keep this high level. Explain that sites depend on anatomy, indication, and clinician judgment, and that the labeled indication is the anchor for treatment planning. Avoid mapping injection points in marketing materials unless your compliance team has reviewed it.
Also address duration and follow-up realistically. People ask, “how long do side effects of Dysport last,” and they also ask how long benefits last. Offer a variability statement and your clinic’s follow-up process, rather than a promise. If concerns persist, document symptom evolution and reassess clinically.
For additional examples of responsible presentation, see Before And After Showcases as internal inspiration for formatting and disclosures.
Clinic Operations Snapshot: Procurement, Storage, and Traceability
Neurotoxin operations should be built for audits, not just convenience. Dysport injections workflow is safest when verification, storage, and documentation are routine. Policies differ across states and facilities, so align your process with medical direction and local requirements.
Clinic workflow snapshot (high level)
- Verify licensure and authorized purchasers.
- Document product selection and intended clinical use.
- Receive inventory and reconcile packing slips.
- Store per manufacturer requirements and facility policy.
- Prepare and administer per clinician training and label.
- Record lot number, expiration, and chart notes.
- Track patient communication and adverse-event follow-up.
When your team trains new staff, create a single storage reference that covers all toxin products you handle. The internal article Store Neurotoxin Products can support that onboarding.
Use a short “receiving checklist” at the point of delivery to avoid missing steps.
- Confirm shipment matches purchase records.
- Inspect packaging for damage or tampering.
- Log lot and expiration immediately.
- Store promptly per written SOP.
- Quarantine discrepancies for review.
MedWholesaleSupplies focuses on supplying authentic, brand-name products for licensed clinical settings.
If your clinic serves multiple service lines, keep a clear separation between procurement hubs and patient-facing education. For example, you might reference product pages for internal cataloging, such as Dysport, Botox, Xeomin, or Azzalure, while keeping clinical decision-making in the chart and training materials.
For broader context on commonly used toxin brands, see Top Botulinum Toxin Brands and the trend-oriented hub Beauty Trends.
Authoritative Sources
For prescribing details, contraindications, and official warnings, use primary sources:
- FDA drug labeling database
- FDA botulinum toxin products safety information
- American Academy of Dermatology
Further reading: align your counseling scripts, consent language, and inventory records so they match the label and your internal SOPs.
This content is for informational purposes only and is not a substitute for professional medical advice.