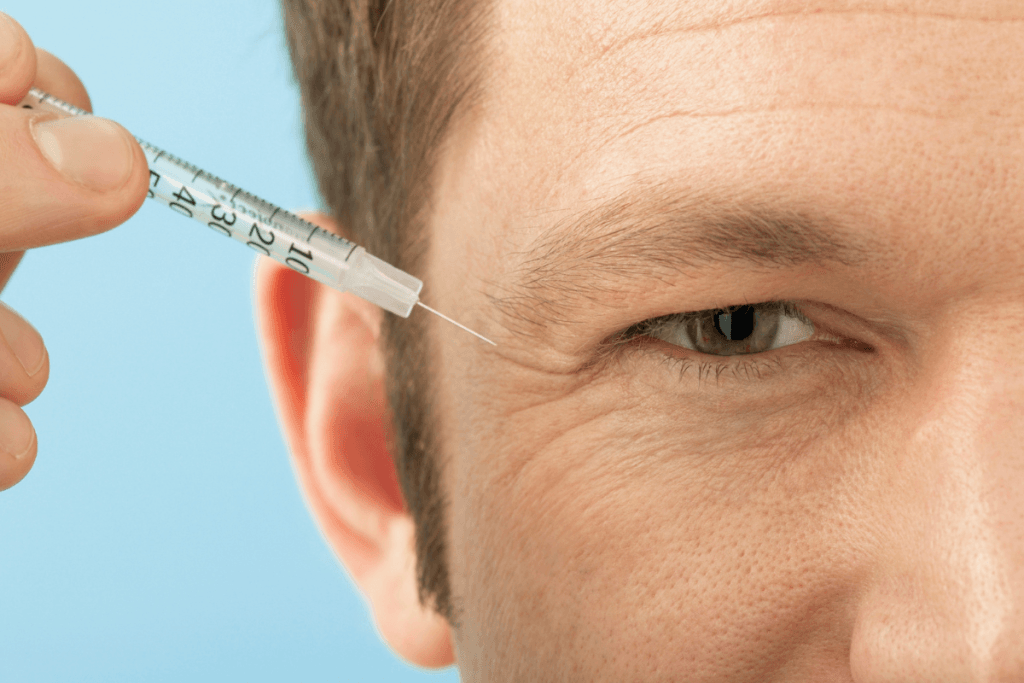
Neurotoxin injectables are now routine in many aesthetic and mixed practices. If your team is evaluating what is dysport, the key is to frame it as a medication class decision, not a marketing choice. Clinics do best when they align on indications, documentation, adverse-event follow-up, and procurement controls before adding another botulinum toxin to the shelf.
Operational details matter as much as clinical familiarity. Consistent photography, standardized consent language, and lot-level traceability reduce avoidable friction later. For practices that rely on US distribution, confirm supply pathways and documentation requirements early.
MedWholesaleSupplies supports licensed clinics and healthcare professionals with clinic-facing access pathways.
Key Takeaways
- Class first: Treat Dysport as a botulinum toxin type A option.
- Units differ: Potency units are product-specific and not interchangeable.
- Document consistently: Photos and charts should be standardized across injectors.
- Plan for safety: Track expected local reactions and escalation red flags.
- Control operations: Keep receiving, storage, and inventory logs audit-ready.
what is dysport and why clinics use it
Dysport (abobotulinumtoxinA) is a prescription botulinum toxin type A injectable. It acts at the neuromuscular junction, where it reduces acetylcholine release and temporarily weakens targeted muscles. In everyday language, it is a “wrinkle relaxer” used to soften expression-driven lines. Like other agents in this class, it is not a dermal filler and does not add volume.
Clinically, you will see it discussed in both therapeutic and aesthetic contexts. Indications vary by country and label, so keep your team aligned on what is authorized in your jurisdiction. When staff field common intake questions, it helps to use consistent terminology across touchpoints. If you need a high-level refresher on mechanism, common uses, and patient-facing expectations, see Dysport Injections How They Work.
From a formulary perspective, Dysport is often considered alongside other botulinum toxin options. Many clinics browse class-level options through a category hub like Botulinum Toxin Category before narrowing to a specific product. For reference within your internal catalog, you can also keep a neutral product record such as Dysport Product Information as part of your medication file.
Setting Expectations and Capturing Before-and-After Evidence
“Before and after” discussions often drive patient satisfaction, but clinics control the risk by controlling the process. Your goal is not to promise an outcome. Your goal is to document baseline findings, align on realistic endpoints, and protect the record. This is especially important when multiple injectors treat the same patient over time.
Many teams start with a simple script that separates outcomes from guarantees. After you explain what is dysport in plain language, move to what you will measure: resting lines versus dynamic movement, symmetry, and functional complaints (for example, tension headaches reported by the patient). If your team uses standardized photo sets, you also reduce subjective “it looked better last time” debates months later.
Photography and documentation basics
High-quality images are operational, not cosmetic. Use the same camera, distance, and lighting. Photograph at rest and with standardized expressions. Document skin conditions that affect interpretation, like tanning, makeup, or recent procedures. Store the images in a consistent location in the EHR or imaging system, with clear encounter labeling and patient consent status.
Consistency matters more than equipment. A mid-range camera used the same way every time is better than an inconsistent setup. If your practice trains new injectors, photography is one of the easiest “competency anchors” to standardize across providers.
Quick tip: Create one photo checklist and attach it to every neurotoxin visit type.
Interpreting changes without overpromising
Patients often search for “before and after pictures” and expect near-identical results. Your documentation should reflect what you can observe and what the patient reports, without implying certainty. If the patient brings outside images, note their source and limitations. Avoid using non-standard angles or filters in your own records, because they complicate comparisons later.
If you want examples of how clinics frame photo outcomes responsibly, review Before And After Result Showcases. Use these as process inspiration, not as outcome promises.
Adverse Effects, Risk Communication, and Follow-Up
Most side effects discussed in clinics are local and procedure-related. These can include injection-site pain, swelling, bruising, headache, or temporary asymmetry. The practical challenge is not listing every possibility. It is building a repeatable method for assessing severity, timing, and functional impact.
When patients ask whether the medication is “safe,” start with framing and documentation. A simple approach is to restate what is dysport, confirm it is a prescription biologic, and explain that risks range from expected local reactions to uncommon serious events described in labeling. Then document what you reviewed, including contraindications or precautions relevant to your setting. Avoid casual reassurance that could conflict with the official label.
MedWholesaleSupplies sources brand-name medical products via vetted distributor channels.
Forehead and periocular considerations to document
“Forehead side effects” is a common theme in patient reviews, but documentation needs clinical specificity. Track baseline brow position, eyelid position, compensatory frontalis recruitment, and any pre-existing asymmetry. If the patient reports visual strain, dryness, or contact lens issues, note it. For periocular areas, record baseline lid function and prior ocular surgery history when relevant.
Do not rely on generic templates alone. A short free-text line about baseline anatomy can prevent confusion later when a patient compares themselves to a friend or a social media image.
How long do side effects last?
Patients routinely ask about duration of bruising or localized tenderness. Give general ranges only, and avoid making time guarantees. If symptoms are worsening, spreading, or affecting breathing, swallowing, or vision, the response should be urgent triage, not reassurance. Track follow-up calls as part of your quality program, and route them consistently to a designated clinician.
Why it matters: Clear follow-up pathways reduce delayed recognition of rare but serious complications.
For a deeper operational view of symptom tracking and patient messaging, see Navigating Dysport Side Effects.
Comparing Botulinum Toxin Type A Options and “Unit” Questions
Clinics often compare Dysport with onabotulinumtoxinA (commonly known by the Botox brand), incobotulinumtoxinA (Xeomin), and other botulinum toxin products. Comparisons show up in patient questions, staff training, and procurement discussions. The core principle is consistent across labels: potency units are specific to each product and cannot be converted with a fixed formula.
This is where the frequent question “100 units of dysport equals how much botox” can create risk. If you need a standard answer for staff, keep it simple: there is no officially interchangeable unit conversion, and dosing decisions must follow the prescribing information and clinical judgment within scope. When staff understand this, they are less likely to repeat social-media ratios as if they were label-based facts.
If your team wants a structured comparison framework, review Botox Vs Dysport Analysis and Top Botulinum Toxin Brands.
| Decision Factor | What to Verify | Why It Affects Operations |
|---|---|---|
| Label indications | Approved uses in your jurisdiction | Shapes consent language and chart templates |
| Unit non-interchangeability | Label statements on potency units | Prevents unsafe “conversion” shortcuts |
| Reconstitution and handling | Product-specific instructions and beyond-use rules | Impacts training, waste, and scheduling |
| Patient perception | Common questions and misconceptions | Reduces chair-time spent correcting misinformation |
It can help to keep neutral reference entries for the products you stock or frequently discuss, such as Botox Product Information, Xeomin Product Information, and Azzalure Product Information. These should support internal verification and staff education, not patient marketing claims.
Clinic Operations: Procurement, Storage, and Traceability
Neurotoxins are high-scrutiny items in many practices because they are prescription-only and workflow-sensitive. Treat them like you treat vaccines or specialty biologics: controlled receiving, clear storage rules, and disciplined documentation. If you run multiple locations, write one policy that covers transfers, chain-of-custody, and who can reconcile inventory.
Procurement teams should also align on supplier qualifications. Confirm that purchasing is restricted to licensed entities, and clarify what documentation you will be asked to provide. Many clinics prefer suppliers that focus on authentic, brand-name inventory and can support audits with appropriate paperwork.
MedWholesaleSupplies focuses on authentic, brand-name medical products for licensed clinical use.
Operational checklist (non-clinical)
- Verify licensure: Keep NPI/DEA and facility records current.
- Control access: Limit ordering authority to named staff.
- Log receiving: Record lot, expiry, and quantity on arrival.
- Store per label: Follow manufacturer storage conditions and monitoring.
- Track use: Document lot numbers in the patient record.
- Reconcile inventory: Investigate discrepancies promptly and document actions.
- Report issues: Use internal incident logs for complaints or excursions.
Clinic workflow snapshot
- Verify licensing and ordering permissions
- Document product selection and intended clinical use
- Receive shipment and reconcile against invoice
- Store under labeled conditions and monitor
- Prepare and administer per prescribing information
- Record lot/expiry and treatment notes in chart
- Monitor follow-up communications and adverse events
Storage and handling details vary by product and local regulation. Use label instructions as your primary reference, then align internal SOPs to match. For a practical overview of storage habits clinics use to reduce waste, see How To Store Neurotoxin Products. If your practice depends on reliable US logistics, confirm receiving hours and escalation contacts in advance.
Patients and staff will still ask about “cost,” often using phrases like “Dysport cost” or “how much is Dysport for forehead.” Keep your internal response focused on variables rather than numbers. Acquisition cost can vary by contracting, minimums, and product mix. Patient charges should reflect your compliance framework, clinical time, overhead, and documentation needs.
Authoritative Sources
When you update policies or staff scripts, use primary sources first. Product labeling is the most defensible reference for indications, contraindications, warnings, and product-specific handling statements. Professional society resources can help with patient communication standards, but they should not replace the label.
The sources below support common clinic questions, including unit non-interchangeability and serious adverse-event warnings that apply to botulinum toxin products.
- FDA Drug Labels Database (search by product name)
- FDA Safety Information for Botulinum Toxin Products
- American Academy of Dermatology: Botulinum Toxin Overview
Further reading can be most useful when it is process-focused. If you are building staff training materials, start with the label, then harmonize your photography, consent, and follow-up workflows across all toxin brands.
This content is for informational purposes only and is not a substitute for professional medical advice.