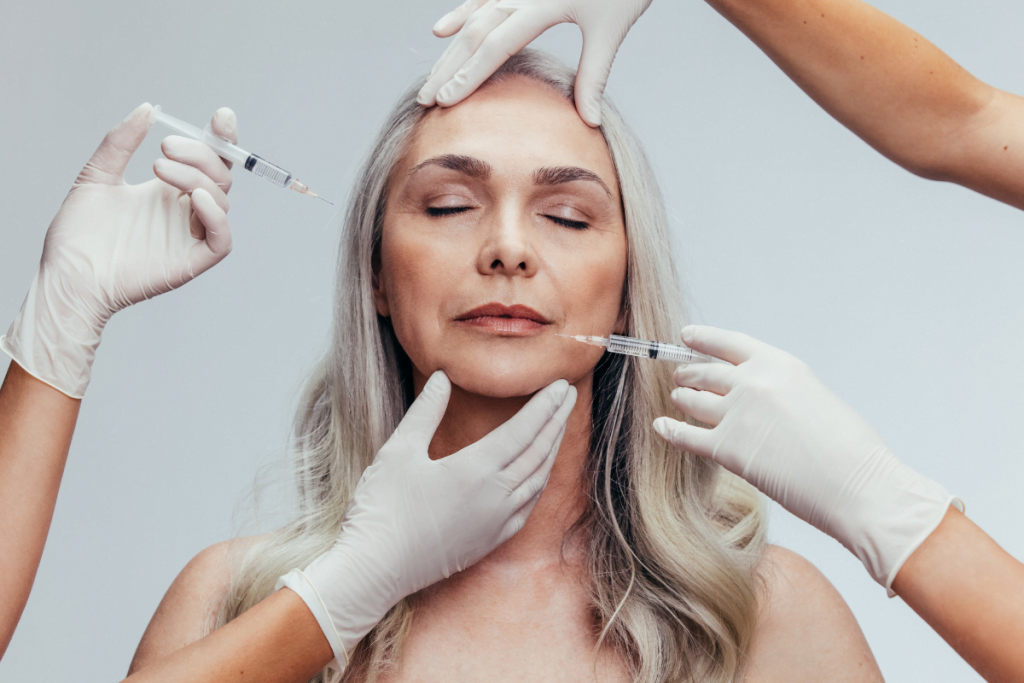
Interest in vegan aesthetic requests is rising in many practices. For clinics, the challenge is practical. “Vegan” can refer to ingredients, manufacturing aids, or animal testing history. These factors rarely align neatly with regulated injectable medicines.
This guide frames vegan-related questions in operational terms. It focuses on what you can verify, how to document discussions, and how to set expectations without overpromising certainty.
Key Takeaways
- Define “vegan” at intake and in consent forms
- Separate ingredient lists from animal-testing realities
- Use manufacturer labeling as the primary source
- Standardize documentation for ethical product requests
- Offer non-injectable alternatives when appropriate
Setting a vegan aesthetic standard in your clinic
Start by deciding what your clinic means when it uses “vegan” language. Some patients mean “no animal-derived ingredients.” Others mean “not tested on animals.” In pharmaceuticals and many medical devices, preclinical testing requirements can complicate “cruelty-free” claims, even when the ingredient list appears compatible with vegan preferences.
Make the definition a policy decision, not an ad hoc conversation. Put it into your intake notes, your patient education materials, and staff scripting. This keeps front-desk, clinical staff, and injectors aligned. It also reduces the risk of inconsistent statements across visits.
When people ask about brand-name neuromodulators or fillers, you can respond with a consistent framework. You can explain what can be checked (labeling, excipients, and manufacturer statements). You can also explain what may be unknowable at the clinic level (certain upstream processing aids or historic testing decisions). That distinction protects trust.
Supplier access is restricted to verified licensed healthcare practices.
If you are updating procurement menus, keep options easy to browse. For example, your team may track availability across hubs like the Botox Category Hub or the Xeomin Category Hub, then validate details against current labeling before use.
Where “Vegan” Can Break Down: Ingredients, Excipients, and Processes
Vegan screening for injectables usually breaks into three buckets. First are active ingredients and excipients (inactive ingredients). Second are manufacturing and processing aids that may not appear on labels. Third is animal testing across development, regulatory submission, or post-market requirements. Your clinic can typically verify the first bucket. The other two often require manufacturer clarification and careful wording in patient-facing materials.
Ingredient transparency matters because some common excipients can be animal-derived or animal-adjacent. Examples include gelatin (a capsule or stabilizer context), lactose (milk-derived sugar), and certain forms of glycerin (which can be plant- or animal-sourced). In addition, some biologic products use human-derived components (for example, human albumin) as stabilizers. Human-derived materials are not “animal-derived” in the usual vegan sense, but they may still raise ethical concerns for some patients. Do not guess. Use the current Prescribing Information or Instructions for Use for the specific NDC/device you stock.
Ingredient lists vs. upstream sourcing
Even when a label lists only synthetic or plant-compatible materials, the origin can still matter to vegan patients. Hyaluronic acid (HA) is a good example. HA can be produced by bacterial fermentation or derived from animal tissue. Many modern HA dermal fillers are described as fermentation-derived, but “fermentation-derived” is still a claim you should verify per manufacturer, per product, and per lot documentation when available. This is where your procurement and clinical education processes intersect.
Why it matters: One unclear ingredient statement can undermine informed consent discussions across your entire aesthetic program.
Common non-vegan flags clinics can screen for
Build a short “flag list” your team recognizes. Staff can scan labeling and manufacturer materials for terms that may trigger vegan objections. These include animal-derived ingredients, but also add-ons around comfort and aftercare. Lidocaine is frequently discussed in dermal filler contexts; the active drug is synthetic, yet patients may ask about excipients, adhesives, or carrier ingredients in topical anesthetics. Similarly, glycerin appears in topical products and can be plant- or animal-derived depending on supplier chain.
Use a structured approach rather than one-off answers. The table below shows a practical way to route common questions to the right source, without making claims you cannot substantiate.
| Clinic Question | Best Primary Source | What You Can Document |
|---|---|---|
| Does this include any animal-derived ingredients? | Current product labeling | Excipients listed; version/date reviewed |
| Is the hyaluronic acid fermentation-derived? | Manufacturer medical information | Written response or referenced statement |
| Was it tested on animals? | Manufacturer policy statement | Policy language and limitations noted |
| Is the numbing product vegan-friendly? | Topical product INCI list | Aftercare product list with flagged ingredients |
Neuromodulators and Fillers: Practical Due Diligence
Patients often ask “is botox vegan” or “is dysport vegan” in the same breath as filler questions. From a clinic standpoint, treat these as separate product classes with separate verification paths. Neuromodulators are prescription biologics with specific labeling and pharmacovigilance obligations. Dermal fillers are regulated medical devices in many jurisdictions and have their own labeling conventions. Your documentation should reflect those differences.
Operationally, your best move is to standardize the questions you ask, then store the answers in a shared internal reference. Keep it version-controlled. Update it when labeling changes or when your supplier changes SKUs. For staff training, point new hires to a stable educational overview such as Exploring Botox Options and a brand landscape summary like Top Botulinum Toxin Brands.
Neuromodulators (botulinum toxin) ethical considerations
Botulinum toxin products raise frequent ethical questions because they are biologic medicines with long development histories. Even if a specific formulation does not list an animal-derived excipient, patients may still object to animal testing requirements or to human-derived stabilizers used in some products. Keep the conversation factual and bounded. Explain that your clinic relies on the approved Prescribing Information, and that “vegan” status is not typically an approved labeling claim for prescription drugs.
If your team needs product-to-product context for training, keep it educational and label-forward. For example, internal reading can include Xeomin Versus Botox Comparison and a mechanism overview such as Dysport In-Depth Look. Avoid turning these resources into simplified “vegan” verdicts. Instead, use them to map what documents to check.
Dermal fillers and common vegan-sensitive inputs
For fillers, vegan screening usually centers on HA origin and additives. When patients ask “is juvederm vegan,” they often mean whether the HA is animal-derived and whether the product includes additives like lidocaine. You can explain that HA is a polysaccharide (a sugar-based polymer) and that many HA products are produced via fermentation, but your clinic should verify claims per manufacturer for the specific SKU and current labeling.
Also consider ancillary supplies and combined treatment plans. If your practice commonly uses neurotoxin plus filler in the same visit, your vegan-related workflow needs to cover both classes and the full tray setup. A helpful internal reference is Combining Neurotoxin and Fillers, used as a process map for documentation rather than a patient promise.
Products are sourced through screened distribution partners, not open marketplaces.
When you discuss clinic inventory, keep the tone neutral. You can point staff to product records such as Botox Product Listing and Dysport Product Listing as internal references, then confirm excipients and labeling from official documents at time of use.
Clinic Communication, Consent, and Documentation
Vegan-related questions are primarily consent and expectation-management issues. Patients are often looking for a clear yes/no answer. In reality, you may only be able to answer parts of the question with high confidence. A good script separates: (1) what the label states, (2) what the manufacturer states outside the label, and (3) what cannot be fully verified by the clinic. Document each category distinctly.
This is also where “cruelty-free injectables” language can create risk. Unless a manufacturer explicitly makes and supports that claim for a specific jurisdiction, your clinic should avoid absolute statements. Instead, describe your process: you review labeling, request ingredient source clarification when available, and disclose limitations about animal testing in regulated product development. This approach aligns with ethical transparency and reduces misinterpretation.
Quick tip: Add a checkbox: “Ethical/vegan ingredient discussion completed; limitations explained.”
Use a structured internal workflow to keep records consistent across providers and locations.
- Verify licensure and authorized purchasing access
- Document the product name, SKU, and labeling version
- Request manufacturer statements for source-sensitive inputs
- Receive and file statements in a shared compliance folder
- Store products per labeled requirements and clinic policy
- Record lot details in the patient chart as required
- Update your internal vegan screening notes on changes
Brand-name items are supplied with provenance documentation on request.
If your procurement team supports multiple locations, standardize the record set you keep for each injectable. At minimum, that includes the current Prescribing Information/IFU, supplier traceability information, and any manufacturer correspondence about animal-derived inputs. For clinics operating with US distribution networks, it is still essential to treat each shipment as a separate verification event, because packaging and inserts can change over time.
Patient-Facing Product Questions: A Checklist for Staff
To keep intake efficient, give staff a consistent checklist. It should work for neuromodulators, fillers, and aftercare products. It should also help staff avoid “off the cuff” answers that later conflict with documentation. The goal is not to persuade patients. The goal is to clearly present what is known, and what is not.
Use the checklist below during consult prep, or when patients message pre-visit. It supports ingredient transparency for injectables and helps your clinic respond consistently to vegan neuromodulators and vegan dermal fillers questions without making unverified claims.
- Define vegan priority: ingredients, testing, or both
- Confirm product class: drug vs device
- Check excipients: albumin, lactose, gelatin, glycerin
- Verify HA origin: fermentation vs animal-derived
- Ask about lidocaine: presence and formulation context
- Record sources: label version and manufacturer reply
- List aftercare: avoid lanolin, beeswax, collagen
- Note limits: animal testing policies may vary
When fillers are the focus, you may want a refresher on comparative labeling language and device indications. An internal primer like Restylane Vs Juvederm can help staff understand how product families are discussed, without turning the conversation into marketing.
Procedure Alternatives That May Fit Vegan Priorities
Some patients pursue “vegan wrinkle treatments” as a broader ethical preference, not a requirement for a specific brand. When injectable constraints feel too uncertain, it helps to discuss adjacent options that may better match the patient’s priorities. This is not a clinical recommendation. It is a pathway to align expectations and informed consent.
Common alternatives include energy-based devices and collagen-stimulating procedures, each with its own consumables. Microneedling can be vegan-friendly in concept, but topical anesthetics, serums, and post-care products may contain animal-derived components. Laser treatments can also align with vegan preferences, but aftercare often includes occlusives or barrier creams where lanolin and beeswax are common. PRP treatment vegan considerations can be different again, because PRP is autologous (from the patient). Even then, you should review accessory products used during processing and post-care. Sclerotherapy vegan considerations may arise due to medication excipients and dressings, so the same documentation habits apply.
As you broaden options, keep the language precise. Rather than claiming a procedure is “vegan,” describe what you have screened: devices used, aftercare products offered, and any ingredient flags. This helps maintain a consistent vegan aesthetic process across services, even when the treatment modality changes. If your practice operates across reliable US logistics pathways for consumables, keep the same verification discipline for non-injectables too.
Authoritative Sources
- Regulatory overview of dermal filler devices: FDA Dermal Fillers (Soft Tissue Fillers)
- Safety communications on botulinum toxin products: FDA Botulinum Toxin Type Products
- Database for approved drug labeling and updates: Drugs@FDA
Documenting ethical preferences works best when your team uses the same sources, the same wording, and the same limits. Review your scripts quarterly, and refresh your internal reference file when labeling changes. A consistent approach reduces risk for staff and improves clarity for patients seeking vegan aesthetic-aligned care.
This content is for informational purposes only and is not a substitute for professional medical advice.