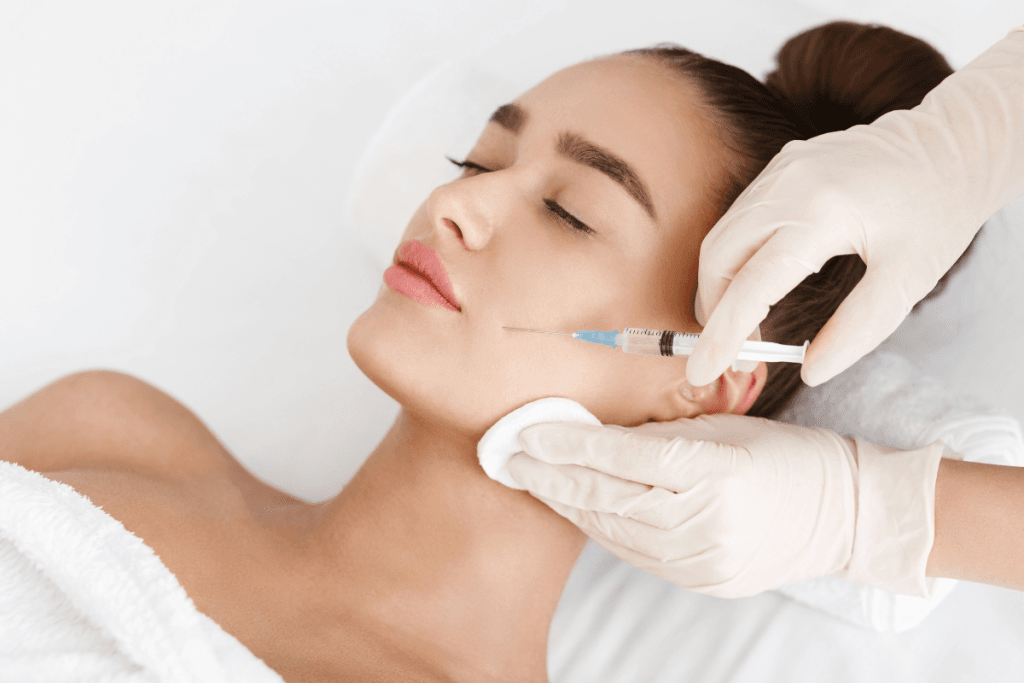
Volume restoration planning is rarely about a single syringe. It is a structured process that weighs anatomy, tissue quality, patient goals, and follow-up capacity. Many clinics use Sculptra as one option within that broader plan, alongside hyaluronic acid (HA) gels and other collagen-stimulating injectables. Operationally, success depends as much on documentation and communication as it does on technique.
This guide is written for licensed healthcare teams. It focuses on practical planning steps, how to interpret “before and after” expectations, and how to set up a clean procurement and tracking workflow.
Key Takeaways
- Plan by region, tissue, and goal, not by “vials” alone
- Use consistent photography and consent for comparison images
- Discuss risk realistically; document baseline and follow-up findings
- Compare product classes by reversibility, texture, and workflow fit
- Expect patient questions like “Sculptra vs Radiesse” and prepare scripts
Sculptra in Volume Restoration Planning: Practical Overview
Sculptra is commonly grouped with biostimulatory injectables. In plain language, it is often discussed as a “collagen stimulator” rather than a gel that simply occupies space. That framing matters because it changes how patients interpret timing, what “results” should look like, and how you document progress. For teams, it also affects inventory planning, follow-up scheduling, and how you handle image requests.
Start by defining the clinical problem you are addressing: true volume loss, contour imbalance, skin quality changes, or a combination. Patients often present with a single aesthetic request, but your intake should translate that request into measurable objectives. Keep your language consistent across consult notes, consent, and post-procedure documentation.
What patients mean by “volume” (and what you should clarify)
Many patients use “volume” to mean projection, lift, or symmetry. Others mean softening of shadows from age-related deflation. Some ask for specific body applications they have seen online, including the phrase “glute augmentation,” even when the conversation should start with candidacy, safety boundaries, and regulatory considerations. If a patient arrives with a plan based on forum posts or influencer content, treat that as an information gap, not noncompliance.
Quick tip: Build a one-page intake addendum that captures goal, region, prior injectables, and photo consent.
Distribution partners may restrict fulfillment to verified licensed facilities and credentialed healthcare professionals.
Interpreting Before-and-After Requests Without Overpromising
Many consults begin with screenshots. Patients search terms like “Sculptra before and after” and expect a predictable visual arc. Your role is to convert that expectation into a documented baseline and a comparison method that is fair, consistent, and clinically defensible. That means standardized photography, stable lighting, and repeatable positioning.
Clinics also need guardrails for what images can and cannot show. “Before and after” pictures compress multiple variables: weight change, makeup, camera angle, and sometimes other procedures. The safest operational posture is to treat photos as supportive evidence, not proof of a specific outcome. When patients request examples for men, jawline definition, periorbital hollows, or “eye” areas, be explicit that facial subunits behave differently and that individualized planning drives the approach.
Photography and consent: make it boring and consistent
Use a repeatable photo protocol and a consent form that covers storage, internal training use, and external marketing use as separate checkboxes. Train staff to capture front, oblique, and profile views with the same focal length and background. If you use 3D imaging, document the device and settings so comparisons remain meaningful. Consider adding a brief note template for “photo limitations” in the chart when a patient supplies outside images from social media.
For deeper context on facial planning vocabulary, you can align your intake language with resources such as Facial Volume Restoration Overview and Types Of Dermal Fillers.
Safety Profile, Adverse Events, and Patient-Reported Concerns
Patients often ask for “side effects photos” because images feel more real than written risks. Your documentation should be equally tangible. Record baseline skin texture, visible asymmetry, prior scars, and any pre-existing nodules. Capture relevant medical history, prior filler type (if known), and timing of past procedures. These details become critical if a patient later attributes a change to a single treatment.
It also helps to address fear-based searches directly and calmly. You may hear statements like “I read a post saying it ruined my face.” Treat that as a signal to slow down and review known risks, uncertainty, and what follow-up looks like in your practice. Avoid debating anecdotal stories. Instead, explain what you can document, what can be monitored, and what is outside the scope of prediction.
Why it matters: When concerns arise, clear baseline notes reduce confusion and rework.
What to include in a clinic-ready adverse event note
If an unexpected reaction occurs, focus on objective details: onset timing, location, appearance, tenderness, systemic symptoms, and any concurrent procedures or illnesses. Note product identifiers where available, including lot number and expiration date. Keep a consistent escalation pathway, including when to involve the supervising clinician, when to consider specialty evaluation, and how to document patient communication. For teams that routinely manage injectable care, create a short internal checklist for chart completion so that the record is complete even when the clinic is busy.
When patients ask about “Sculptra side effects,” emphasize that all injectables can have risks and that long-term issues are best discussed with reference to official labeling and professional guidance rather than forums.
Many suppliers source brand-name products through vetted distributors to support authenticity and traceability checks.
Comparing Biostimulatory and Gel Fillers in Planning
Comparison questions come in many forms: “vs filler,” “vs Radiesse,” or “vs Juvederm.” Your planning framework should compare classes, not marketing claims. Operationally, the most useful decision factors are texture and spread characteristics, reversibility considerations, and how predictable the immediate effect is. It is also practical to consider staff familiarity, training needs, and how you handle post-procedure follow-up.
Procurement teams may also be asked about “Sculptra cost.” Rather than quoting numbers in a clinical conversation, focus on what drives resource use: the need for multiple visits in some plans, the amount of product used varying by anatomy and goals, and the time required for documentation and follow-up photography. For clinical education refreshers, see PLLA Vs Filler Comparison Guide and CaHA Vs PLLA Overview.
| Planning factor | Biostimulatory (PLLA-type) | CaHA-type | HA gel fillers |
|---|---|---|---|
| Primary planning intent | Often selected for gradual change and collagen signaling | Often selected for structure and tissue support feel | Often selected for immediate shape and contour |
| Expectation setting | Emphasize staged assessment and consistent photography | Discuss firmness and region-specific palpability | Discuss swelling variability and refinement visits |
| Correction flexibility | Plan conservatively; avoid “all at once” mental models | Plan by plane and avoid overcorrection narratives | Consider reversibility discussions where applicable |
| Inventory implications | May require tighter lot tracking across visits | Often managed like other injectables with lot control | Broader SKU variety can complicate stocking |
When patients focus on body contour trends, keep the conversation grounded in safety boundaries, anatomy, and realistic limitations of non-surgical approaches. If you need a starting point for how clinics discuss that demand, review Non-Invasive Butt Lift Context alongside your local scope-of-practice rules.
If you are comparing specific catalog items for training alignment, you can cross-reference education with product families such as Radiesse 3 mL and similar SKUs used in your formulary planning.
Clinic Workflow and Documentation Checklist
Injectables live at the intersection of clinical care and regulated product handling. Even when specific requirements vary by jurisdiction, a consistent workflow reduces errors. Your process should cover verification of authorized purchasers, receipt and inspection, lot and expiration capture, storage per manufacturer instructions, and chart linkage. This is also where you decide who can access inventory and how waste is recorded.
If your clinic supports multiple services, it helps to map which teams touch product at each step. Some practices keep injectable workflows separate from other services listed under hubs such as Dermal Fillers and Mesotherapy to reduce cross-handling and miscounts. If you rely on US distribution for continuity, document your receiving and reconciliation steps so coverage staff can follow them.
Documentation checklist for procurement and audit readiness
- Authorized purchaser file: licenses and clinic credentials on record
- Receiving log: date, quantity, inspection notes
- Lot and expiration: captured at receipt and at use
- Storage notes: per labeling, with access controls
- Chart linkage: product identifiers tied to encounter
- Photo consent: versioned forms and retention policy
- Adverse event pathway: escalation roles and templates
Clinic workflow snapshot
- Verify: facility and clinician credentials
- Document: approved purchasers and storage SOPs
- Procure: match SKU to formulary and indications
- Receive: inspect packaging and record identifiers
- Store: follow manufacturer instructions and access control
- Administer: document site, technique summary, and follow-up plan
- Record: lot/expiry, photos, and patient communications
For inventory alignment with your formulary, some clinics keep a reference link to Sculptra 2 Vials alongside internal handling and charting templates, without turning the chart into a product brochure.
Authoritative Sources
When you need to verify regulatory status, labeled use, contraindications, and handling instructions, use the official label as your primary reference. Online reviews and discussion boards can highlight questions patients are asking, but they should not substitute for labeled information or professional training standards.
These sources can support clinic policy writing, consent language reviews, and staff education refreshers. Confirm the most current versions before updating SOPs.
Recap for Practice and Procurement Teams
A strong volume restoration plan is repeatable: consistent intake, standardized photos, clear consent, and rigorous product traceability. Patient-facing questions often cluster around comparisons, “reviews,” and body-contour trends, including the phrase “Sculptra for buttocks.” Your best response is a calm framework that separates goals, safety boundaries, and what follow-up will look like in your clinic.
Clinic teams also benefit from supplier processes that serve licensed healthcare customers and maintain brand-name sourcing through screened distribution channels. If you depend on reliable US logistics, keep a contingency plan for inventory continuity and documentation coverage across staff rotations. For deeper reading, revisit Role Of Poly-L-Lactic Acid and How Calcium Hydroxylapatite Works.
This content is for informational purposes only and is not a substitute for professional medical advice.