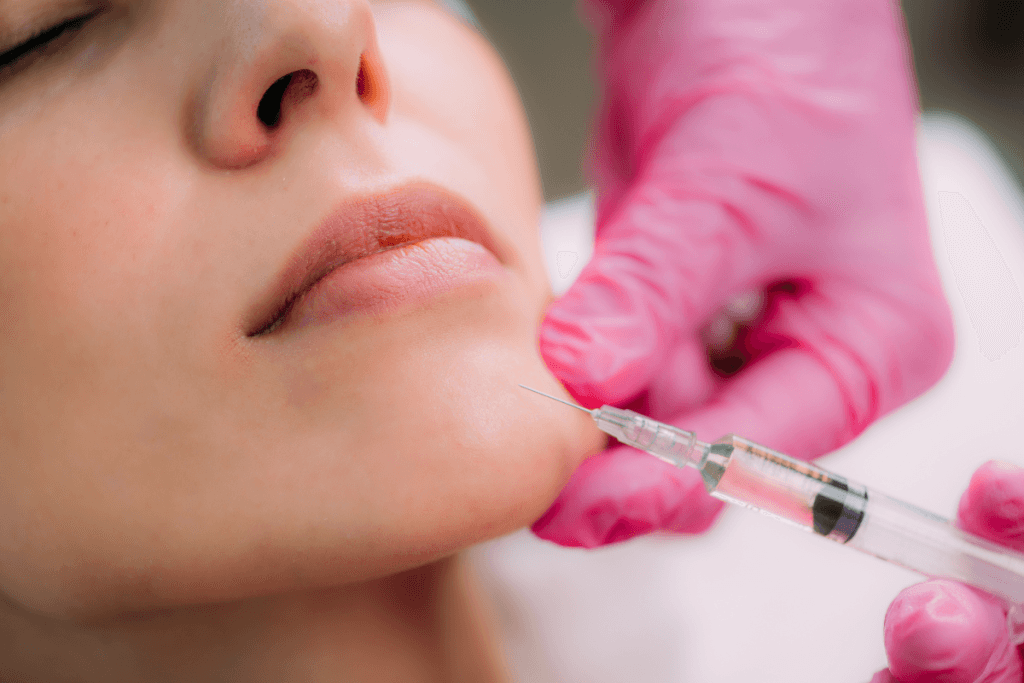
Hyaluronic acid (HA) dermal fillers remain a core tool for facial contouring. Products differ in gel structure, lift, spread, and handling. For many practices, the day-to-day question is operational: how to match a “mid” filler to common goals, document the plan, and manage risk. This guide uses celosome mid with lidocaine as a reference point for those clinic discussions, without substituting for local labeling or training.
Instead of focusing on marketing descriptors, it helps to translate them into measurable needs. Think: tissue depth, desired projection, mobility of the area, and the patient’s tolerance for discomfort. Your documentation should show that logic. Your procurement and storage steps should support traceability.
Key Takeaways
- Map product choice to plane, mobility, and contour goal.
- Use rheology terms to communicate handling expectations.
- Standardize consent, photos, and lot/expiry documentation.
- Plan for complications, including reversal pathways.
- Compare “mid/soft/deep” within the same clinical framework.
Where a Mid-Viscosity HA Filler Fits
A mid-range HA gel is often positioned between “soft” (more spread, subtle refinement) and “deep” (more projection, structural support). In practice, that translates into how the filler integrates under dynamic movement, and how much shape it holds in areas like the midface, folds, or perioral regions. The right match can reduce rework and improve chart clarity, even before outcomes are considered.
Most HA fillers rely on cross-linking (chemical bonds between HA chains) to increase durability and change flow. Cross-link density, particle/gel architecture, and manufacturing process influence elasticity (how well the gel resists deformation) and cohesivity (how well it “sticks together” as a mass). If your team needs a refresher on HA fundamentals, the overview in Hyaluronic Acid In Aesthetic Medicine can help align terminology across injectors and coordinators.
Supplier access is typically limited to verified licensed healthcare professionals.
Filler cohesivity and G prime explained
Clinics often hear rheology terms without a clear decision link. G′ (G prime) is commonly used as a shorthand for elasticity, which relates to “lift” and shape retention. A higher-elasticity gel may provide more structural support, while a lower-elasticity gel can spread more easily for superficial refinement. Cohesivity describes how the gel behaves as a unit; more cohesive gels may stay together and define edges, while less cohesive gels may feather more smoothly. These are not guarantees of results. They are practical descriptors you can use during product selection, staff training, and charting.
celosome mid with lidocaine in Clinic Practice
This product name signals two operational points: it is an HA dermal filler, and it includes a local anesthetic (lidocaine) intended to reduce injection-site discomfort for many patients. From a workflow standpoint, that affects consent language, adverse-event counseling, and how you compare patient experience across product lines. It also influences how you schedule follow-up, since comfort and swelling can affect early patient perception.
When your practice evaluates an HA gel that includes lidocaine, treat it like any other regulated component in the chart. Ensure your consent and allergy screening address amide anesthetics where appropriate, and document what was used. For deeper reading on the clinical rationale, see Lidocaine In Filler Procedures.
Lidocaine as a procedural adjunct
Lidocaine in a prefilled syringe is often framed as a comfort feature. Operationally, it also changes how staff explain sensation during and immediately after treatment. Some clinics find that comfort improvements reduce mid-procedure pauses and allow more consistent technique. Still, lidocaine does not remove the need for slow, deliberate placement and clear safety protocols. If a patient reports escalating pain, blanching, or visual symptoms, treat that as a safety signal rather than “normal discomfort.” Align this messaging across clinical and front-desk staff, so patient calls are triaged consistently.
Assessment and Indication Mapping for Common Areas
Before you decide whether celosome mid with lidocaine (or a comparable “mid” HA option) belongs in a protocol, define the problems you are treating in plain language. “Fold softening,” “cheek contour,” “lip definition,” and “jawline sharpness” each imply different depths and different tissue movement. A standardized assessment template helps: facial asymmetry notes, skin quality, movement patterns, and prior filler history (including complications and dissolving events).
Common clinic use-cases that teams often map to a mid-range gel include nasolabial folds, marionette lines, selected midface contouring, and some lip work where structure and spread must be balanced. For general background on volumization planning, your staff can review Facial Volume Restoration, and then apply that framework to the products your clinic actually stocks.
Mid-dermis filler application: plane and goals
“Mid-dermis” is used loosely in conversation, so define it in training. In many techniques, the target plane is chosen to match the goal: superficial refinement may prioritize smooth spread, while deeper placement may prioritize support and projection. A mid-depth approach often aims for contour change with reduced visibility and palpability, but the risk profile still depends on the region. Your protocol should specify how staff confirm anatomy landmarks, how they document plane selection, and what the expected short-term appearance changes are. “Before and after” photography also matters operationally: take consistent angles, lighting, and facial expression to support follow-up discussions and chart review.
Technique and Device Choices: Needle vs Cannula
Clinics evaluating celosome mid with lidocaine should separate product selection from technique selection. Needle and cannula choices can change bruising patterns, speed, and control, but neither is inherently “safer” in every setting. The key operational question is whether your injectors are trained, supervised where required, and aligned on a consistent approach for each facial zone.
Device choice also affects how you standardize your inventory. You may stock different cannula gauges and lengths, multiple needle sizes, antiseptics, and emergency supplies. Tie these items to a written procedure set, then audit it. If you want broader context on filler categories and typical use-areas, Types Of Dermal Fillers is a helpful staff primer.
Cannula vs needle: practical trade-offs
Needles may offer precise placement with minimal entry points in skilled hands, while cannulas can reduce the number of skin punctures and may navigate along tissue planes differently. Those practical differences matter most in mobile areas, in patients prone to bruising, and when you need broad fanning rather than point placement. Your chart should capture the device used and the anatomic entry site(s). This supports continuity of care and helps if a patient presents later with swelling, nodules, or delayed inflammatory reactions.
Common pitfalls:
- Vague charting: unclear plane or region documented.
- Rushed setup: incomplete aseptic field preparation.
- Inconsistent photos: lighting and angles vary widely.
- Overreliance on comfort: ignoring warning symptoms.
- Weak follow-up: no standardized check-in window.
Safety, Contraindications, and Reversal Planning
Any HA filler workflow should begin with safety screening and a clear plan for adverse events. When your protocol includes celosome mid with lidocaine, treat contraindications broadly: known hypersensitivity to components, active infection at or near the injection site, and situations where elective procedures should be deferred. Final decisions depend on the product’s official instructions for use and your local regulations.
Expected short-term reactions can include erythema (redness), swelling, tenderness, and bruising. Delayed issues can include nodules, inflammatory reactions, or infection. Rare but serious complications include vascular compromise and visual symptoms. Your clinic should have a written escalation pathway, including emergency referral criteria, on-call coverage expectations, and documentation templates for incident reporting.
Why it matters: Delayed recognition of vascular events can increase the risk of harm.
Reversal planning is part of risk management, not a marketing point. HA fillers are unique in that they can often be dissolved with hyaluronidase when clinically indicated. Your team should know where hyaluronidase is stored, who can access it, and how events are documented. Avoid improvisation; follow training, product labeling, and local guidance.
Clinic Operations: Verification, Storage, and Records
Operational discipline matters as much as injection skill. If you carry celosome mid with lidocaine alongside other HA fillers, your inventory system should support traceability and audits. That includes a consistent receiving process, a defined storage area, and a clear way to record lot and expiry in both inventory logs and patient charts. Policies vary by jurisdiction, so confirm your local recordkeeping requirements.
Many practices also prefer to consolidate purchasing through suppliers that emphasize brand-name sourcing and distributor vetting. MedWholesaleSupplies, for example, is positioned toward licensed clinics and healthcare professionals and focuses on authentic, brand-name products sourced via screened distributors.
Clinic documentation checklist
- License file: keep current credentials on record.
- Receiving log: date, lot, and expiry captured.
- Storage checks: follow labeled conditions and access controls.
- Patient chart: product name, lot, and site documented.
- Photo set: consistent angles and consent status.
- AE pathway: escalation notes and incident record template.
- Waste handling: record partials per clinic policy.
- Recall readiness: searchable lot-level inventory report.
Quick tip: Make lot/expiry entry mandatory before closing the note.
To keep product selection organized, it helps to use browseable hubs for your team. You can review the Dermal Fillers assortment and narrow by the Hyaluronic Acid Fillers category when updating protocols. Some clinics also maintain a short comparison list of commonly stocked SKUs, such as Juvederm Volift With Lidocaine, Restylane Defyne, or Stylage L Bi-Soft With Lidocaine, depending on their preferred handling characteristics and local availability.
Inventory is typically obtained through distribution partners that have been screened for legitimacy.
How to Compare Mid vs Soft vs Deep Options
Product naming is inconsistent across manufacturers. “Soft,” “mid,” and “deep” can reference different gel technologies, recommended planes, and handling. For clinic operations, the goal is not to crown a “best” filler. It is to build a repeatable selection pathway that new staff can learn and experienced injectors can audit.
A simple comparison tool helps during onboarding and when you add a new brand. If you need cross-brand context, Restylane vs Juvederm is a useful example of how practices frame differences without relying on anecdotes alone.
| Label Tier (Common) | Typical Operational Goal | Common Documentation Focus | What to Confirm |
|---|---|---|---|
| Soft | Subtle refinement and blending | Surface smoothness and mobility notes | Superficial plane guidance on label |
| Mid | Balanced contour with controlled spread | Plane selection rationale and photo consistency | Rheology fit for the target area |
| Deep | Structure, projection, and support | Risk counseling and anatomy landmarks | Depth guidance and complication plan |
When comparing options, keep the discussion grounded in (1) labeled indications and plane guidance, (2) injector familiarity, (3) patient comfort strategy, and (4) how your clinic will monitor and document outcomes. If you maintain multiple HA lines, limit overlap where possible. A smaller, well-understood formulary can reduce errors in storage, selection, and charting. Product authenticity and traceability remain central to that approach.
Authoritative Sources
For high-level safety and regulatory framing, use primary sources and major professional organizations:
- FDA overview of dermal fillers and safety considerations
- American Academy of Dermatology on soft tissue fillers
- American Society for Dermatologic Surgery on dermal fillers
In day-to-day clinic operations, focus on consistent assessment language, traceable records, and a rehearsed complication pathway. Use product labels and training to refine technique details, and keep your formulary aligned with what your team can document reliably.
This content is for informational purposes only and is not a substitute for professional medical advice.