Neurotoxin procurement is rarely just a “brand choice.” It affects consultation language, documentation, storage routines, and how your team explains results. This briefing focuses on azzalure botox as an example of botulinum toxin type A used for targeted wrinkle reduction. It is written for licensed healthcare providers who need an operational view, not patient-facing marketing.
You will see the same clinical themes that apply across products: unit conventions, reconstitution steps, injection-site mapping, adverse event readiness, and traceability. Where labeling or approvals vary by country, default to your local product information and regulations. For broader brand context, you can also review the site’s roundup of Popular Botulinum Toxin Brands.
Key Takeaways
- Units are product-specific and not dose-convertible across brands.
- Plan injection sites with anatomy, symmetry, and complication avoidance in mind.
- Set expectations using ranges; onset and duration vary by patient and technique.
- Standardize documentation: lot numbers, reconstitution notes, and consent language.
- Use a consistent handling workflow to reduce waste and mix-ups.
Where Azzalure Fits in a Neurotoxin Portfolio
In day-to-day practice, “Botox” is often used as shorthand for any botulinum toxin injection. Operationally, that shorthand can create avoidable errors. Different botulinum toxin type A products have different unit definitions, labeling, and presentations. Your protocols should name the specific product administered and match the local label.
Many clinics organize sourcing and SOPs by a category view rather than by one brand. A simple place to orient staff is a hub like the Botox Category or the site’s Botox Tag, then drill down into product-specific documents. This reduces reliance on memory when onboarding new injectors.
Mechanism of action, in plain terms
All botulinum toxin type A products work by reducing acetylcholine release at the neuromuscular junction. That dampens muscle contraction and can soften the appearance of expression lines over time. In aesthetic clinics, the practical implication is that outcomes depend on both product handling and technique: accurate placement, dose distribution within a muscle, and a plan that respects individual anatomy.
It also helps to use plain-language synonyms in your consult scripts. “Neuromodulator” and “botulinum toxin injection” may be clearer than a single brand name. That approach can reduce confusion when you discuss alternatives or manage backorders.
Access is generally restricted to licensed healthcare facilities.
Regulatory status and labeled indications for Azzalure can differ by region. Keep your patient-facing materials aligned to what is approved locally. When a request falls outside the label, treat it as an off-label conversation with appropriate consent and documentation standards.
Azzalure Botox in Practice: What Clinics Should Review
When teams evaluate a new neurotoxin, the biggest risks are rarely clinical surprises. They are workflow mismatches: unit confusion, inconsistent dilution habits, and uneven counseling language across injectors. Before you adopt any product broadly, align on what “standard” looks like in your clinic and how you will audit it.
Start with your internal recordkeeping and inventory controls. If you maintain a product list for staff, keep it specific and link to training resources. For example, your internal page can reference the Azzalure Tag and related clinical primers, while your procurement notes can reference the supplier’s documentation requirements.
Units, labeling, and documentation
Clinicians will see searches like “azzalure botox units” and “azzalure dosage guidelines” from staff and patients alike. Treat those phrases as a cue to standardize language: units are product-specific, and they are not interchangeable across toxins. If you use more than one brand in your practice, build this rule into training, chart templates, and consent discussions.
Operationally, the chart should capture the product name, total units used, injection pattern at a high level, and the lot number. Your adverse event plan should also reference the exact product administered. This matters when you need to compare outcomes, investigate a complaint, or respond to a pharmacovigilance report.
Why it matters: Unit confusion is a preventable root cause of dosing errors.
| Decision factor | What to verify | Why it matters |
|---|---|---|
| Local label and approvals | Indications, contraindications, and required materials | Aligns consent language and reduces off-label drift |
| Unit convention | Unit definition and charting standard | Prevents accidental “conversion” between products |
| Reconstitution steps | Aseptic technique, diluent type, and timing per label | Supports consistent results and safer handling |
| Training plan | Injector competency, supervision, and refresh cadence | Reduces variability across providers |
| Traceability | Lot tracking, storage logs, and wastage recording | Improves audit readiness and incident review |
Teams also ask “azzalure botox made in” when they review packaging. The safest approach is simple: verify the country of manufacture, MAH details, and labeling language on the carton and official product information for your market. Avoid relying on secondhand summaries, which can be outdated or region-specific.
If you maintain multiple toxins, a structured comparison worksheet helps. For deeper brand-specific overviews, see the site’s summaries on Botox Overview, Dysport Alternative Guide, and Xeomin Uses And How It Works.
When sourcing matters, use suppliers that can support authenticity documentation for brand-name inventory.
Technique Planning: Injection Sites, Anatomy, and Common Requests
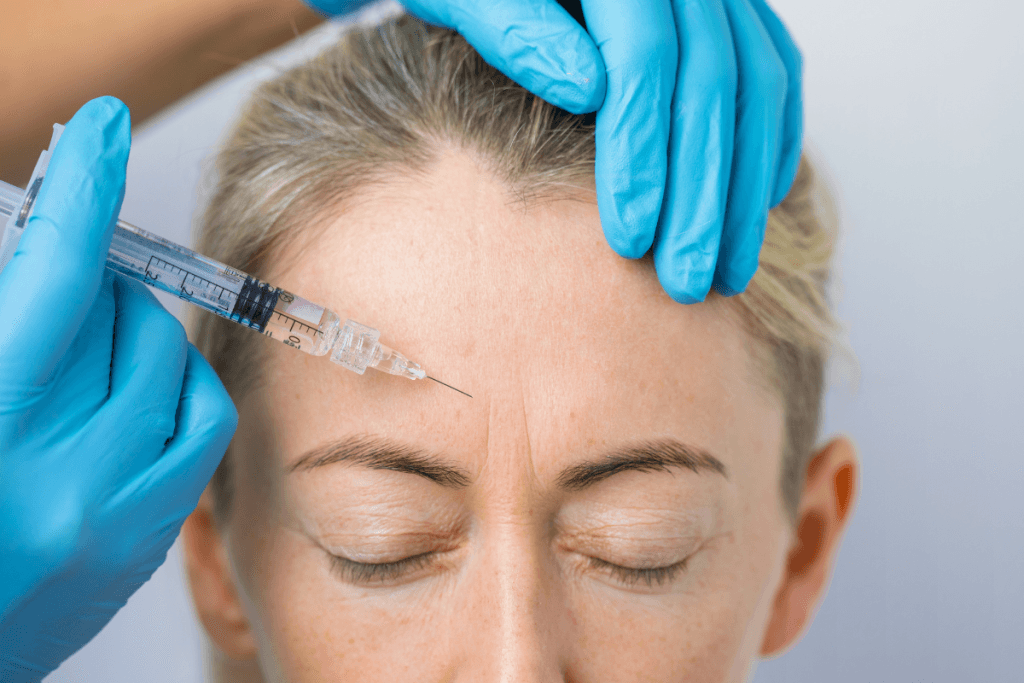
Searches for “azzalure injection sites” usually reflect a practical need: teams want a repeatable mapping approach. The safest way to operationalize injection planning is to standardize three steps: (1) assess facial dynamics at rest and animation, (2) map target muscles and risk zones, and (3) document the plan in a way another injector can interpret.
This section is not a dosing guide. Follow your local label, training, and institutional policies. Still, clinics can reduce variability by agreeing on how to describe patterns and how to capture them in the EMR. Even simple diagram templates can reduce rework at follow-ups.
Upper-face mapping (glabella, forehead, lateral canthus)
Upper-face treatments dominate many aesthetic schedules. Requests for azzalure for glabellar lines, azzalure for forehead lines, and azzalure for crow’s feet are common because those regions show high expression-driven change. Operationally, your consistency depends on the pre-injection assessment. Confirm baseline brow position, eyelid show, pre-existing asymmetry, and compensatory frontalis activity. A standardized photo set and short animation video can be valuable for internal review.
When you standardize documentation, avoid overpromising based on “before and after” marketing. Use the photos to support clinical continuity and patient understanding. In internal training, focus on anatomy, spread risk, and your clinic’s escalation pathway if function changes occur.
Lower-face considerations and off-label requests
Lower-face requests, such as azzalure for masseter reduction or azzalure for gummy smile, often involve nuanced anatomy and variable goals. In many jurisdictions these uses may be off-label. That makes consent language, realistic expectation setting, and follow-up planning especially important. In your workflow, consider adding an “off-label” documentation flag and a second-check step for newer injectors.
Lower-face injections can also increase the risk of unwanted functional effects if placement is imprecise. Keep your complication readiness practical: identify who evaluates concerns, what timeframe you aim for triage, and how you document symptom onset and progression.
In technique discussions, azzalure botox can be treated like any other toxin: plan, map, document, and review outcomes.
Onset, Duration, and Setting Expectation Ranges
Clinics see recurring questions like azzalure onset time, azzalure results timeline, and how long does azzalure last. The operational goal is not to quote a single number. It is to give a range and explain the drivers of variability: baseline muscle strength, injection placement, total dose per area, prior exposure, and individual biology.
Most practices describe neurotoxin onset as occurring over days, with results evolving as muscle activity decreases. Duration is often discussed in terms of months rather than weeks, but it can be shorter or longer depending on patient factors and treatment pattern. When you set expectations, separate “first noticeable change” from “maximal effect” and “return of movement.” That reduces unnecessary early touch-up requests.
Quick tip: Use the same timeline language across staff to avoid mixed messages.
For clinic tracking, consider a simple outcomes framework that does not drift into marketing. Your team can log patient-reported satisfaction, clinician-assessed symmetry, and any functional concerns. If you collect azzalure botox before and after photos, use consistent lighting, head position, and expression prompts. That makes internal audits more meaningful than informal “azzalure botox reviews” shared in chat threads.
Authentic, brand-name products should come through channels that prioritize traceability.
Safety, Contraindications, Aftercare, and Complication Readiness
Search terms like azzalure side effects, azzalure safety and contraindications, and azzalure complications and risks indicate what patients and staff worry about most. Your role is to ensure counseling is consistent, documentation is complete, and escalation pathways are clear. Always use your local product information as the primary reference for contraindications, warnings, and adverse reaction reporting.
In general, clinics counsel on local injection-site reactions and the possibility of unwanted muscle effects in adjacent areas. More serious systemic effects are uncommon but are part of class-wide safety discussions for botulinum toxin products. Make sure your consent process covers expected effects, possible unwanted effects, and what constitutes an urgent concern. Keep the language plain, then document that it was reviewed.
A practical aftercare plan should be written and consistent. Many clinics include elements often searched as azzalure aftercare instructions, such as avoiding vigorous manipulation of treated areas and monitoring for unexpected functional changes. Protocols vary by practice and product label. Train staff to give the same instructions in person and in written follow-up notes.
Build “what we do if” guidance into team training. When a patient calls with a concern, your response should not depend on who answers the phone.
Pitfalls clinics can prevent
- Unit “conversion” assumptions across brands
- Inconsistent reconstitution documentation
- Vague charting of injection patterns
- Under-triage of functional complaints
- Overconfident promises about duration
In brand comparisons, clinicians may ask about azzalure vs botox or azzalure vs dysport. Keep those discussions grounded in labeling, handling, and your injector experience. Avoid implying equivalence of units or outcomes without reference to official guidance. For region-specific alternatives, some clinics also review information on products such as Bocouture Guide.
Procurement, Storage, and a Simple Clinic Workflow Snapshot
Neurotoxin problems often start before the injection room. Mix-ups happen during receiving, stocking, and reconstitution. A clear workflow reduces waste and supports traceability. If your team is updating cold-chain logs or stocking practices, the storage primer How To Store Neurotoxin Products can support staff training, alongside your facility policies.
Clinics also need a sourcing model that fits compliance requirements. MedWholesaleSupplies focuses on serving licensed healthcare professionals and clinics with brand-name products through vetted distribution partners. Many practices also prefer reliable US logistics to simplify receiving and documentation.
For handling, treat azzalure botox like any prescription neurotoxin: verify the shipment, document identifiers, store per label, and control access. When you reconstitute, follow the official instructions for azzalure dilution and reconstitution, including aseptic technique, labeling of the prepared vial or syringe, and discard timing per the product information.
Checklist: documentation and handling steps to standardize
- Verify supplier documentation and licensure requirements
- Log product name, strength, and presentation
- Record lot number and expiry at receiving
- Maintain temperature and access logs per policy
- Use a consistent reconstitution note template
- Label prepared syringes with time and preparer
- Chart administered product and total units used
- Document adverse events and follow-up actions
If you are comparing options for your formulary, you may also look at product pages for internal reference, such as Azzalure Product Page or Dysport Product Page. Keep clinical decision-making tied to the local label and your governance process.
Authoritative Sources
For further reading, review your local product information and align it with clinic SOPs. A short internal training session on units, documentation, and triage can prevent most avoidable issues.
This content is for informational purposes only and is not a substitute for professional medical advice.