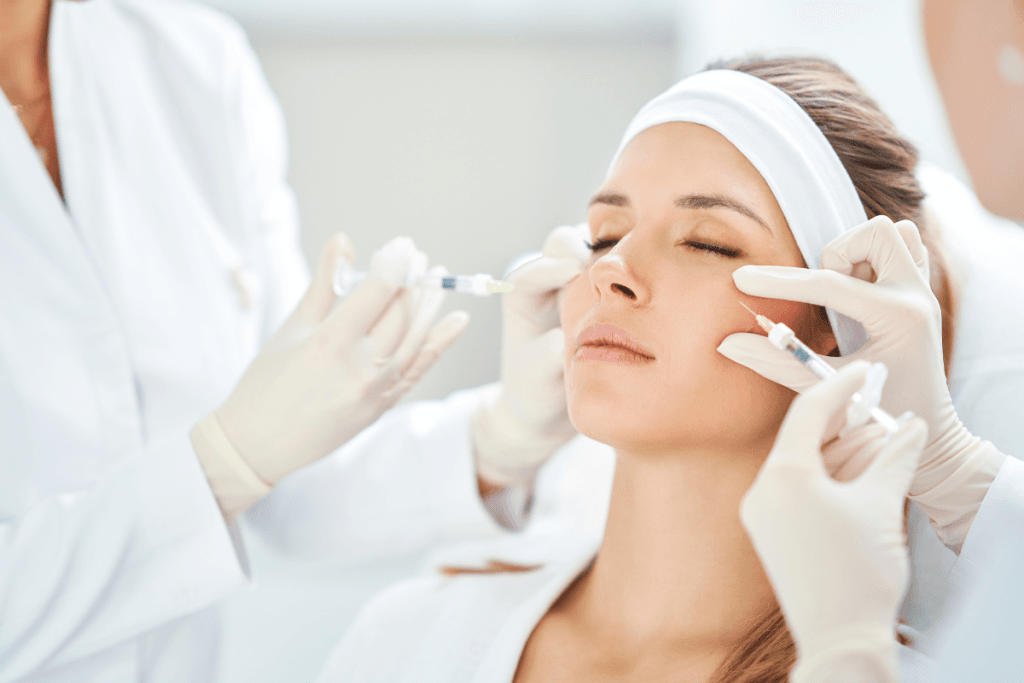
Skin boosters have become a routine add-on in many aesthetic clinics. Teams want consistent protocols, clear documentation, and predictable downtime counseling. This guide reviews how Nucleofill treatment may fit into a regenerative injectable menu, without turning it into a product sheet. It focuses on operational decisions, patient communication frameworks, and practical safety screens.
You will see frequent questions in consults and intake notes. Clinicians ask about polynucleotide skin boosters, mapping of injection points, and what “before and after” usually means in real-world follow-up. Practice managers also ask about traceability, storage expectations, and how to verify supply channels.
Key Takeaways
- Position skin boosters by goal, not by brand name.
- Plan documentation for injection sites, consent, and lot traceability.
- Set expectations for gradual change and variable maintenance needs.
- Screen for contraindications and define clear escalation pathways.
- Compare classes using mechanism, downtime, and workflow fit.
Nucleofill treatment: Clinic-Facing Overview
Nucleofill is commonly discussed as a polynucleotide (nucleic-acid fragment) skin booster. In practice, clinics consider it when a patient wants improved skin quality rather than structural volume. Think “surface and tissue support” goals such as texture, hydration feel, and radiance. Some practices also evaluate these products for challenging zones, including periorbital skin and neck and décolletage, where subtlety matters.
Your first task is defining where a polynucleotide approach fits among your current services. Many clinics already offer hyaluronic acid (HA) boosters, collagen-stimulating devices, and neuromodulators. A polynucleotide booster is often positioned as a series-based service with clinical photography and standardized follow-up intervals. That structure matters as much as the syringe selection.
MedWholesaleSupplies supplies verified healthcare accounts and typically restricts access to licensed professionals.
Why it matters: Clear service definition reduces rework when results are subtle and gradual.
When teams discuss “nucleofill candidacy,” it helps to separate three concepts. First is skin quality concern (dullness, crepey texture, post-acne changes). Second is tolerance for short-term injection reactions like swelling and bruising. Third is patient preference for a natural-looking shift rather than immediate “filler-like” change.
For product-specific details, keep your staff aligned on one rule. Use the manufacturer’s IFU for indications, contraindications, and handling steps. If your clinic uses multiple Nucleofill variants, keep a one-page internal matrix for “zone,” “expected feel,” and “who should not receive it,” without adding dosing instructions.
For broader context on how these services are discussed in clinics, see Skin Boosters Overview and the brand-adjacent background in Nucleofill Medium Article.
What Polynucleotide Skin Boosters Are (and Aren’t)
Teams sometimes describe boosters as “injectable skincare.” That shorthand can confuse patients and new staff. A skin booster is not primarily a volumizer, and it is not a topical. It is an injectable approach that aims to improve skin quality markers over time, with outcomes that can be harder to quantify than lift or projection.
It also helps to be explicit about what a booster does not replace. It does not substitute for sun protection, topical retinoids, pigment-directed therapy, or scar revision plans. In acne-scar consults, for example, it may be discussed alongside subcision, energy-based devices, and targeted resurfacing, depending on clinician assessment and local standards.
Mechanism of action, in plain terms
Polynucleotide products are generally described as biologic-origin polymers that may influence the local tissue environment. In clinic language, that often means “supporting dermal quality” rather than “filling.” The proposed nucleofill mechanism of action is usually framed around tissue hydration support and signaling that can be associated with fibroblast activity and extracellular matrix maintenance. Because published evidence and regulatory status vary by country and product, keep claims conservative in your materials. Avoid promising collagen percentages or fixed timelines. Instead, document observable endpoints you can measure: standardized photos, patient-reported texture changes, and consistency of downtime across sessions.
Patients may cross-shop polynucleotides against HA boosters and mesotherapy-style cocktails. If you already provide HA-based services, keep a clear educational distinction between a “hydration-focused HA booster” and a “polynucleotide skin booster.” Your intake form can include both terms, plus plain-language synonyms, to reduce confusion.
For category-level browsing of related injectables, your team can reference Dermal Fillers and Hyaluronic Acid Fillers as internal hubs.
Protocol Planning: Mapping, Technique, and Documentation
Consistency is the main operational risk in booster programs. Two clinicians can describe the same service but execute it differently. That variation makes it difficult to interpret nucleofill strong reviews or “before and after” discussions across providers. Your goal is not identical artistry. It is repeatable documentation and clear boundaries for technique choices.
For Nucleofill treatment planning, many clinics build a standardized template that records the target region, laterality, depth plan, and a simple map of nucleofill injection points. The “points” concept is less about memorizing a pattern and more about ensuring reproducible coverage. Templates also help when a patient switches providers within the same practice.
What to capture in the chart (without overcomplicating it)
Start with a consistent pre-procedure set. Include baseline photos with the same lighting and distance. Document the patient’s primary goal in their words, plus your clinic’s defined endpoint (for example: “skin texture and radiance”). Record the product name as labeled, lot number, and expiration date. Add a brief injection-site map, using either a face diagram or a short written grid (cheek, periorbital, neck). Finally, standardize aftercare instructions in the chart, even if you also provide a handout. This reduces variation when staff rotate or when follow-ups happen by phone.
Technical choices should follow training, anatomy, and the IFU. Keep technique discussion in internal SOPs and competency checklists. If you need a refresher on injection safety frameworks, route staff to Safety Protocols Guide.
When clinics add an eye-area service, align language carefully. “nucleofill eyes treatment” is a common search phrase, but periorbital injections require advanced anatomy knowledge and a conservative approach. Maintain a clear scope statement for what your clinic offers, and document patient counseling in detail.
Expected Course: Results Timeline, Downtime, Aftercare
Patients often ask for nucleofill before and after photos at the first touchpoint. Clinics can set expectations by explaining that many boosters show gradual change, not instant transformation. The nucleofill results timeline can also vary by baseline skin quality, region treated, and whether other procedures occur in the same period.
When you discuss how long does nucleofill last, avoid fixed promises. Instead, explain the concept of a treatment series and the possibility of nucleofill maintenance sessions. Make it clear that maintenance depends on your clinic’s protocol, patient preference, and observed response, and that schedules can differ across providers and jurisdictions.
Downtime counseling should be specific and operational. “nucleofill downtime” usually refers to short-term injection-site reactions rather than prolonged recovery. Prepare patients for typical nucleofill swelling and bruising patterns, and document any prior history of easy bruising or post-procedure inflammation.
Quick tip: Use the same photo timing at every follow-up visit.
Aftercare instructions should be simple, consistent, and compliant with your local standards. Many clinics provide a one-page summary and reinforce it verbally. If you want a structured framework for post-injection counseling language, see Post-Treatment Care Essentials.
For internal review, consider a quarterly audit of “treatment before and after” documentation. Track whether photos are usable, whether aftercare was recorded, and whether follow-up timing was consistent. This improves clinical governance and reduces dissatisfaction driven by inconsistent evidence.
Safety Screen: Contraindications, Adverse Effects, and Red Flags
Most injectable complications are not unique to one brand. They are process-related: patient selection, aseptic technique, anatomy, and response to early warning signs. Your screening should therefore be standardized across booster types, including polynucleotide products and HA boosters.
Before scheduling Nucleofill treatment, clinics commonly review allergy history, prior filler complications, active skin infection near the treatment area, and relevant systemic conditions that may affect healing. Your nucleofill contraindications list should come from the product IFU and your clinic’s medical director policies. When in doubt, document uncertainty and defer the decision to the responsible prescriber or clinical lead.
Staff should be prepared to discuss nucleofill side effects in plain language. Expected reactions can include transient erythema (redness), tenderness, and localized swelling. Less common but more serious events may involve infection or vascular compromise, depending on injection region and technique. Define “call us” versus “seek urgent care” criteria in your patient instructions and in staff scripts.
For periorbital work, risk tolerance should be even tighter. Your clinic should have a clear escalation pathway for eye symptoms after any facial injection, regardless of product class.
- Inconsistent consent language across providers
- Poor photo standardization at follow-up visits
- Incomplete lot and expiry documentation
- Underestimating bruising impact on schedules
- Mixing multiple injectables without a plan
Finally, address regulatory questions with precision. Patients and staff may ask “is nucleofill fda approved” or repeat “nucleofill fda approved” as a blanket claim. In the US, regulatory status depends on the specific product and indication. Keep your clinic’s statements factual, and direct staff to verify status using official sources rather than marketing materials.
Comparing Options: Strong Variants, Rejuran, and HA Boosters
Comparisons can derail consults when they turn into brand debates. Bring the conversation back to decision factors that matter operationally. For Nucleofill treatment, that means mechanism class, target region, expected texture change, and compatibility with your clinic workflow.
Within the line, “nucleofill strong” and “nucleofill strong plus” are often discussed as variants selected for different tissue needs. Do not infer differences from the name alone. Confirm nucleofill strong ingredients and labeling details directly from the manufacturer documentation you have on file. This protects the clinic when staff answer questions or write after-visit summaries.
Patients may also ask about “nucleofill strong vs rejuran” or “nucleofill or profhilo.” These comparisons usually reflect a desire to understand product class rather than exact equivalence. Keep your explanation anchored in the type of biostimulation versus hydration strategy, typical downtime expectations, and the kind of endpoint you measure.
| Decision factor | Polynucleotide booster | HA skin booster / hydrobooster | Other injectable revitalizers |
|---|---|---|---|
| Primary goal | Skin quality support over time | Hydration feel and fine-line softening | Varies by formula and protocol |
| What patients “see” | Gradual texture and tone change | Often subtle, sometimes quicker glow | Usually gradual and variable |
| Clinic documentation focus | Photos, texture notes, site mapping | Photos, hydration notes, site mapping | Ingredient/lot traceability, consent |
| Common operational risk | Overpromising visible change | Confusing with volumizing filler | Inconsistent protocols across staff |
For staff who want quick product-line references during training, use internal links sparingly and neutrally. Examples include Nucleofill Strong 1.5 mL, Rejuran HB, and Sunekos. For HA-style hydrobooster background reading, see Viscoderm Hydrobooster Guide and Sunekos Treatment Advances.
If you offer an eye-area booster service, keep the discussion product-agnostic. You can reference a dedicated listing such as Nucleofill Eyes in internal staff materials, while keeping public-facing language within verified claims and local regulations.
Clinic Operations: Sourcing, Verification, and Stock Control
Most complications in aesthetic procurement are preventable. They come from unclear sourcing, incomplete records, and inconsistent inventory practices. For Nucleofill treatment programs, traceability matters because outcomes are assessed across multiple visits and sometimes multiple providers.
MedWholesaleSupplies sources brand-name medical products through distributor-vetted channels.
Clinic workflow snapshot
- Verify licensed account credentials and authorizations
- Document product name, lot, and expiry on receipt
- Store per manufacturer instructions and clinic SOP
- Dispense/administer under clinical governance policies
- Record injection sites, reactions, and follow-up plan
- Retain traceability for audits and incident review
Build a simple “chain of custody” habit. Match what arrives to what was expected, then record it once in a consistent place. If your clinic runs multiple locations, align naming conventions so inventory systems do not split one product into multiple entries.
Use procurement language that supports compliance. Avoid informal phrases like “equivalent” unless you can substantiate it. When you discuss authenticity, keep it procedural rather than promotional.
MedWholesaleSupplies may request documentation to support product provenance and account eligibility.
Finally, decide how your clinic will handle patient education assets. Create one approved handout per booster class. Include what is normal after injections and what is not. Keep the focus on monitoring and follow-up, not guarantees.
Authoritative Sources
For US regulatory framing and safety communications, use neutral primary sources rather than marketing summaries.
- FDA overview of dermal fillers and approved uses
- FDA medical device databases for verification
- American Academy of Dermatology cosmetic safety basics
In summary, treat booster services as a program, not a single visit. Standardize mapping, photos, consent language, and traceability. When questions arise about approval status or comparisons, anchor answers in product-specific documentation and regulator resources.
This content is for informational purposes only and is not a substitute for professional medical advice.