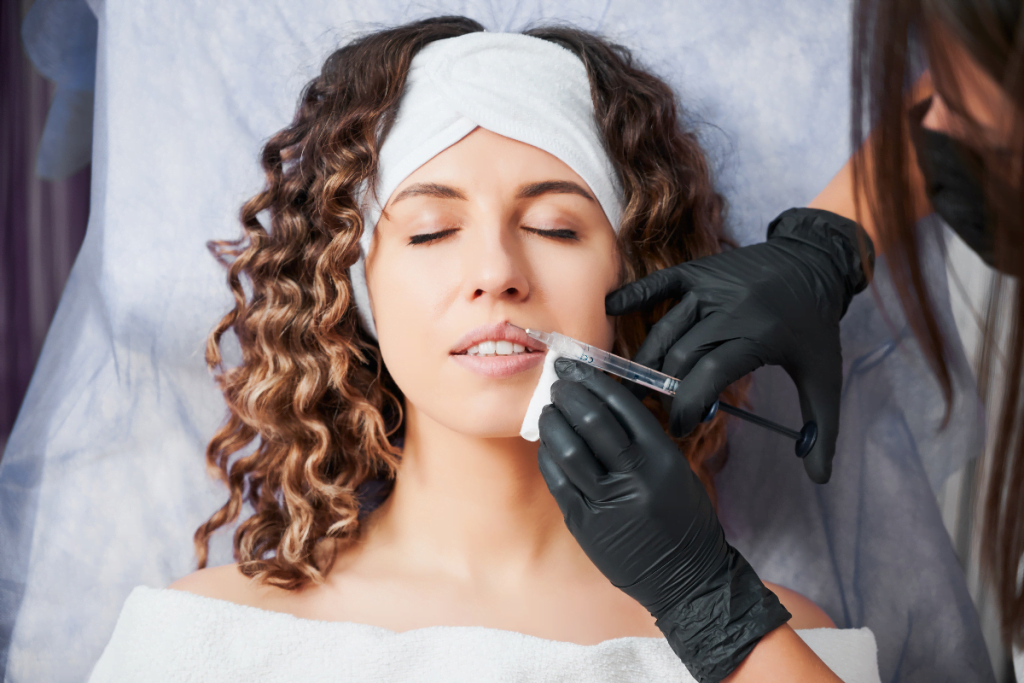
Interest in radiesse for lips often comes from two pressures. Clinicians want durable structure. Patients want a “natural” look with predictable downtime. The gap is that calcium hydroxylapatite (CaHA) behaves differently than hyaluronic acid (HA) gels, especially in mobile tissue. This guide summarizes where CaHA may fit, what to document, and how to set operational guardrails.
Because lip augmentation is highly visible, small technique and product differences become big satisfaction drivers. You can reduce risk by standardizing how you assess anatomy, capture baseline photos, and verify product sourcing before it enters inventory.
Key Takeaways
- Clarify on-label vs off-label use early
- Standardize photos, consent, and aftercare handouts
- Compare CaHA and HA by reversibility and feel
- Budget for total episode costs, not unit cost
MedWholesaleSupplies supplies only to licensed clinics and healthcare professionals.
radiesse for lips: What Clinics Should Know First
Radiesse is a CaHA-based dermal filler. Many teams first encounter it through cheek, jawline, or hand rejuvenation workflows. Interest then extends to perioral contouring and lip-related requests. Before you operationalize any approach, confirm your local labeling, training standards, and medical director policy. Indications and permitted injection planes vary by jurisdiction and product labeling.
From an operations view, treat this topic as a “special scenario” rather than a default lip menu item. Your protocol should define when CaHA is considered, who can perform the service, and which documentation is required. You will also want a clear pathway for managing typical post-procedure concerns, including swelling, asymmetry during early healing, and patient-shared photos on social platforms.
For a refresher on scope and selection principles, start with Lip Augmentation Overview and your internal competency checklist.
Where Calcium Hydroxylapatite Fits in Lip Augmentation
Most lip augmentation requests are solved with HA fillers. HA products are familiar, have a wide viscosity range, and may be reversible with hyaluronidase (per clinical judgment and local protocols). CaHA has different handling and tissue response characteristics. That difference can be an advantage in selected cases, but it also changes your risk framing.
Clinics often discuss “radiesse filler” as a collagen-supporting option. In practice, you should translate that into operational questions. What is the target outcome: border definition, subtle projection, perioral support, or a broader facial plan? What is the tolerance for palpability, early firmness, or unevenness during remodeling? Align the conversation with what your clinicians can reproducibly deliver.
Material and Tissue Response
CaHA fillers combine a gel carrier with calcium hydroxylapatite microspheres. The gel provides immediate space-occupying effect, while the microspheres may support neocollagenesis (new collagen formation) over time. That “biostimulatory” framing matters for counseling, because the look can evolve as the carrier dissipates and tissue response occurs. In lips, mobility, thin mucosa, and patient sensitivity to small surface changes can amplify perceived irregularities. For a deeper mechanism review, see Calcium Hydroxylapatite Primer.
Operationally, document your rationale for choosing CaHA versus an HA product. That rationale helps with internal QA reviews and supports consistent messaging if a patient compares outcomes to HA-based lip treatments.
Patient Selection, Risk Framing, and Consent
When a patient asks about radiesse for lips, your first task is translation. Many are searching for “longer lasting” or “more natural,” not specifically CaHA. Others are reacting to social content, including radiesse reviews and forum summaries like “radiesse filler reddit.” Build a brief intake script that captures the motivation, prior filler history, and tolerance for uncertainty in early healing.
Define inclusion and exclusion criteria at the clinic level. Your criteria should be based on clinician training, anatomic assessment, and known filler safety principles. Avoid ad hoc decision-making during a consult. Instead, use a structured pathway that includes baseline exam, photo set, consent elements, and a plan for follow-up communication.
Anatomic And Safety Considerations
Lip and perioral injections intersect with vascular risk, swelling, and patient expectations around symmetry. Even experienced injectors can face complaints when early edema (fluid swelling) distorts landmarks. Your consent process should clearly address common post-procedure changes, potential complications, and escalation steps. Keep the language plain. “Vascular compromise” can be explained once as reduced blood flow risk. Also document relevant history, including prior filler types, prior adverse reactions, and any recent procedures that may affect swelling or bruising.
Why it matters: Consistent consent reduces confusion when early swelling does not match the final look.
Before-and-After Photography and Swelling Communication
Most dissatisfaction starts with mismatched expectations, not product failure. Patients search “radiesse before and after” and expect a single dramatic image to predict their outcome. Clinics can counter this by standardizing how you capture and present images. Use consistent lighting, camera distance, head position, and a neutral facial expression. Store the photo set in the chart with a timestamp.
Build a simple “swelling expectations” handout that your staff can reuse. Patients also search for radiesse swelling pictures and may assume that the worst-looking image is typical. Your handout should explain that swelling varies by patient and procedure, and that early asymmetry is common across lip fillers. If you discuss “radiesse swelling time,” keep it non-numeric unless you are using label-supported language; emphasize variability and clinic follow-up channels instead.
Products are sourced as brand-name inventory through vetted distributor channels.
For internal training on image standards and outcome counseling, your team can cross-reference Lip Filler Duration Guide and Types Of Lip Fillers to align terminology across staff roles.
How to Compare CaHA, HA, and Biostimulatory Options
Clinicians and patients often ask for simple head-to-head answers. In reality, the useful comparison is about properties and workflows. The search terms radiesse vs sculptra and radiesse filler vs hyaluronic acid reflect different product families and different “time horizons” of effect. Your clinic can make this easier by defining a few decision factors that appear in every consult note, regardless of product chosen.
When you compare brands in conversation, keep it neutral and feature-based. For example, questions like radiesse vs juvederm for lips or radiesse vs juvederm voluma are often proxies for “soft vs structural” and “lip vs midface.” Similarly, patients may ask about revanesse lips, or compare revanesse lips vs juvederm, revanesse lips vs restylane kysse, or revanesse lips vs versa. Your documentation should reflect the clinical reason for the chosen category, not internet popularity.
How To Compare: Practical Decision Factors
- Reversibility pathway: consider HA-specific reversibility
- Tactile goals: softness versus structured support
- Downtime tolerance: swelling and bruising variability
- Follow-up plan: touch-up expectations and monitoring
For staff education on broader facial use cases, including references patients may cite like “radiesse before and after jawline” or “radiesse before and after neck,” keep a curated reading list such as Facial Volume Filler Types and CaHA Vs PLLA Comparison.
Procurement, Verification, and Inventory Controls
Adding a new filler option is not only a clinical decision. It changes purchasing, storage, and traceability work. If your team is evaluating radiesse for lips as a service line, set the same controls you use for other injectables: defined supplier criteria, documented receiving steps, and lot-level recording in the patient chart. Follow manufacturer instructions for storage and handling, and keep processes consistent across locations.
In many clinics, “radiesse cost” conversations start with unit acquisition price. A better approach is total episode cost. Include staff time for consult, photo capture, consent, follow-up messaging, and any remedial visits. Also consider the cost of carrying inventory and expiration risk. This mindset helps you compare “radiesse fillers cost” versus HA options without over-focusing on a single line item.
Clinic Workflow Snapshot (Non-Clinical)
- Verify account: confirm license and authorized users
- Source selection: document distributor and product identifiers
- Receive goods: inspect packaging and record lots
- Store inventory: follow labeled storage requirements
- Chart use: record product, lot, and site notes
- Review outcomes: log issues for QA trending
Quick tip: Use one standardized intake form for all filler consults.
MedWholesaleSupplies serves verified clinical accounts and distributes brand-name products via screened suppliers.
When you need to reference examples in purchasing discussions, keep links informational and limited. See Dermal Fillers Category for a browseable list and the editorial hub at Dermal Fillers Articles. For product identifiers used in documentation, examples include Radiesse 3 mL Product, Stylage Lips Plus, and FILLMED Lips Soft. Policies vary by supplier and jurisdiction, so confirm your receiving and recordkeeping requirements.
If your sites depend on US distribution, assign one owner for reconciliation and lot logging.
Authoritative Sources
When counseling patients or writing protocols, anchor key statements to primary sources. Use the official labeling for indications, warnings, and handling requirements. For adverse event discussions, reference regulator safety communications rather than social summaries. That keeps your consent language consistent and defensible across providers.
These sources are a practical starting point for dermal filler risk framing and patient education materials. They are also useful for staff onboarding and annual compliance refreshers.
- FDA dermal fillers overview and safety information
- American Academy of Dermatology patient-facing filler basics
In summary, radiesse for lips discussions work best when you treat them as a protocol decision. Standardize assessment, photography, and consent language first. Then align purchasing and documentation to support consistent care delivery.
This content is for informational purposes only and is not a substitute for professional medical advice.