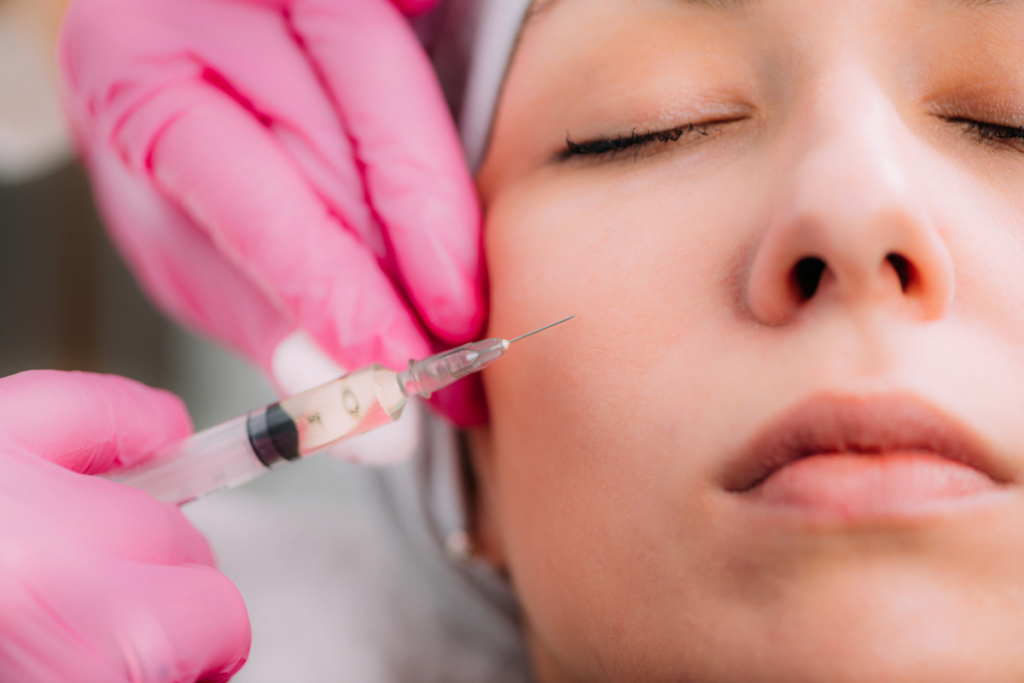
Procurement and clinical teams often begin with a simple query: what is stylage filler, and how does its range fit everyday aesthetic practice. The answer is less about one product and more about how a brand’s variants map to different tissues, technique preferences, and risk tolerance.
This overview focuses on operationally useful details. It covers how to think about product families, common treatment areas, safety and regulatory questions, and what to document for traceability. For general background across brands, you can also browse the broader Dermal Fillers assortment as a reference point for categories and naming conventions.
Key Takeaways
- Think in product families, not single SKUs
- Match gel behavior to tissue and movement
- Plan documentation for photos and lot tracking
- Verify regulatory status by country and indication
- Maintain protocols for adverse event escalation
Understanding what is stylage filler in Clinic Practice
In clinical terms, Stylage is a line of hyaluronic acid (HA) dermal fillers. HA is a naturally occurring glycosaminoglycan (water-binding sugar) found in skin and connective tissue. In aesthetic medicine, HA gels are used to restore volume, soften lines, or refine contour in selected facial areas. Some versions are also positioned for skin hydration and texture improvement, rather than structural lift.
For clinics, the practical question is how a given variant handles in tissue. That includes gel cohesivity, elasticity, and how it integrates in superficial versus deeper planes. It also includes whether a formulation contains an anesthetic such as lidocaine, which can affect patient comfort workflows and consent language. If you want a broader framework for comparing families of injectables, the overview in Types Of Dermal Fillers can help standardize your internal terminology.
Distribution is typically limited to verified licensed healthcare accounts.
Why this matters for operations: “A dermal filler line” can represent multiple handling profiles. When your team aligns on vocabulary and indication boundaries, you reduce errors at ordering, storage, and charting steps. That consistency also supports training, peer review, and adverse event documentation.
Stylage Filler Types: How the Range Is Usually Structured
Clinics looking beyond marketing language often ask again, later in the evaluation process, what is stylage filler in terms of variants and how they differ. Most HA filler lines separate products by intended depth, structural support, and softness, often reflected in naming that suggests “softer” versus “more volumizing” options.
In day-to-day selection, it helps to translate names into a few functional buckets: fine-line correction in more superficial tissue, mid-depth correction for folds and moderate volume, higher support for contour and projection, and skin-quality boosters for hydration. Within the Stylage family, you may see multiple sizes and “with anesthetic” options that support consistent patient experience planning. For example, clinics may stock a mid-range option such as Stylage M Bi-Soft With Lido alongside a lip-focused format like Stylage Lips Plus Bi-Soft With Lidocaine and a hydration-oriented option such as Stylage Hydro Max Bi-Soft.
Linking names to rheology (how gels behave)
Rheology describes how a material deforms and flows under force. In filler practice, clinicians often use rheology as shorthand for “will this gel hold shape” and “how will it move with expression.” A higher-support gel may be preferred when projection or contour is the priority. A softer, lower-support gel may be preferred in highly mobile or thin-skinned areas where visibility and palpability are concerns. These are not absolute rules, and training and technique vary by injector. Still, documenting your team’s preferred mapping between product tiers and anatomical regions can reduce variation across providers and locations.
Quick definitions:
- Elasticity: resistance to deformation
- Cohesivity: tendency to stay together
- Viscosity: resistance to flow
- Integration: blending within tissue planes
- Anesthetic option: lidocaine-containing formulation
For clinics that use anesthetic-containing fillers, it is worth aligning on counseling and intake language. Consider linking your internal SOP to a primer such as Lidocaine In Dermal Filler Procedures so staff use consistent terminology across consent, labeling, and post-visit documentation.
Common Treatment Areas and Planning Considerations
When teams review before-and-after portfolios, they may informally describe “best use cases” like cheeks, lips, tear troughs (under-eye hollowing), and nasolabial folds. A useful clinic-level approach is to define what success looks like in each area in plain language, then map that goal to a gel category and a documentation plan. That keeps discussions grounded in outcomes you can assess and record.
For example, lip enhancement is often discussed in terms of border definition, hydration, or volume. Cheek work is often framed around contour support and midface balance. Under-eye correction is frequently approached conservatively because of thin skin and a smaller margin for visible irregularities. For deeper folds, clinicians may use more structural support, depending on anatomy and movement. If your clinic wants a practical overview of lip-specific workflow and expectations, the article Stylage Lip Filler For Clinics provides a focused operational lens.
Photography and “before and after” documentation
Even when patients ask about “before and after,” your clinic’s main use of standardized photography is clinical documentation and quality improvement. Set consistent lighting, patient positioning, and facial expression instructions. Record the product variant, lot number, injection sites (high-level), and any immediate reactions. Build a routine follow-up documentation window that matches your practice standards and local regulations. This is also where you can track a results timeline in a neutral way, noting swelling trends or settling patterns without promising a specific outcome.
In intake and checkout, many clinics standardize post-procedure guidance. A staff-facing reference like Post-Treatment Care Essentials can help keep instructions consistent across providers, while still deferring to the product’s official instructions for use (IFU) and the clinician’s judgment.
Safety Profile: Side Effects, Contraindications, and Complications
As with other HA-based injectables, side effects can include transient swelling, bruising, tenderness, erythema (redness), and palpable firmness. Clinics should also be prepared for less common but higher-risk complications, including delayed nodules, infection, hypersensitivity reactions, and vascular compromise. The practical safeguard is not a single step, but layered controls: patient screening, product selection, aseptic technique, and clear escalation protocols.
Contraindications and precautions depend on the specific product and jurisdiction. In general, clinicians review prior filler history, autoimmune or inflammatory conditions where relevant, active skin infection at the site, bleeding risk considerations, and any known sensitivity to formulation components (including anesthetic, if present). Your team should align on how these factors are documented, and where exceptions or deferrals are recorded in the chart.
Why it matters: Delayed recognition of vascular compromise can increase tissue injury risk.
Many clinics prefer authenticated, brand-name inventory for traceability.
Also plan ahead for expectation management. Patients may interpret normal swelling as a “result.” Your documentation and counseling language should separate immediate post-procedure changes from later appearance. When patients ask whether “is stylage a good filler,” the most defensible answer is process-based: selection and outcomes depend on anatomy, technique, and the specific product’s labeling, not the brand name alone.
Regulatory Status and “FDA-Approved” Questions
Clinic teams will encounter questions like “is stylage filler fda approved” during consults and while updating marketing or consent materials. The operational response is to treat regulatory status as product- and country-specific. “FDA-approved” is not a blanket statement for an entire class, and indications matter. The safest workflow is to confirm the exact product name, intended use, and the regulator’s current position before staff use that language.
In the U.S., many internationally marketed fillers are not cleared or approved for aesthetic indications, even when they are available elsewhere. If you operate with US distribution channels, ensure your compliance team can substantiate any regulatory statements in patient-facing materials. The same caution applies when patients ask “is elasty filler fda approved” or “is rejeunesse filler fda approved.” Do not generalize from reputation, packaging, or informal reviews.
A practical way to handle “list of fda approved fillers” requests is to point staff to the regulator’s own resources, then document what you relied on when updating clinic language. For neutral reference, see the FDA’s overview in Dermal Fillers (Soft Tissue Fillers).
Finally, separate regulation from quality. A product’s legal status does not automatically answer whether it fits your protocols, training, and documentation standards. Keep those decision points explicit in your internal review notes.
Clinic Operations: Sourcing, Documentation, and Inventory Workflow
When your staff returns to the basics—what is stylage filler in your formulary, and what does it replace or complement—build the answer into a controlled workflow. That includes vendor verification, receiving checks, and traceability from storage to patient record. Policies vary by organization and state, but the structure should be consistent enough that any trained team member can follow it.
Clinic workflow snapshot:
- Verify licensure and account access
- Confirm product name and variant
- Receive and inspect packaging
- Record lot and expiration details
- Store per IFU requirements
- Document use in the chart
- Reconcile remaining inventory
Quick tip: Keep lot numbers and expiry dates in your EHR or inventory log.
For centralized browsing and standardized naming, some clinics use a single internal link to a product hub such as Dermal Fillers Product Hub when building order sets and onboarding documents. If your model depends on reliable US logistics, add a receiving checklist that focuses on integrity and documentation rather than speed claims.
Reputable suppliers source inventory through screened, accountable distribution partners.
At minimum, your documentation set usually includes invoices or packing slips, lot and expiration records, and a clear chain from inventory to administration. For multi-provider practices, define who can remove stock, where it is logged, and how discrepancies are investigated. Keep staff language neutral and aligned with the IFU for storage conditions and contraindication statements.
How to Compare Stylage With Other HA Fillers
Comparison questions often show up as “stylage filler vs juvederm” or “stylage filler vs teosyal.” For clinic decision-making, a fair comparison focuses on decision factors you can validate: official labeling and indications (by jurisdiction), available variants (including anesthetic options), handling characteristics your injectors can describe consistently, and your ability to maintain traceable sourcing.
Also consider how you will train to consistency. If you introduce a new line, define which providers can use it first, what proctoring looks like, and how you will standardize charting language for areas like cheeks, lips, and nasolabial folds. A structured discussion is more valuable than informal “reviews,” because it ties product choice to governance.
For deeper reading within your team, use side-by-side explainers like Stylage Vs Juvederm and broader context pieces such as Restylane Vs Juvederm. To keep staff scripts accurate, it also helps to review common misconceptions in Myths And Misconceptions About Dermal Fillers.
Authoritative Sources
For neutral, regulator-led background on device classification, adverse events, and approved indications, review the FDA resource: Dermal Fillers (Soft Tissue Fillers).
For clinician- and patient-facing safety overviews that can inform counseling language, see the American Academy of Dermatology’s information hub: Dermal Fillers.
Further reading can be most useful when it answers one operational gap. If your team is evaluating duration expectations and follow-up documentation, review Long-Lasting Natural Results with your clinic’s own outcomes tracking standards in mind.
This content is for informational purposes only and is not a substitute for professional medical advice.