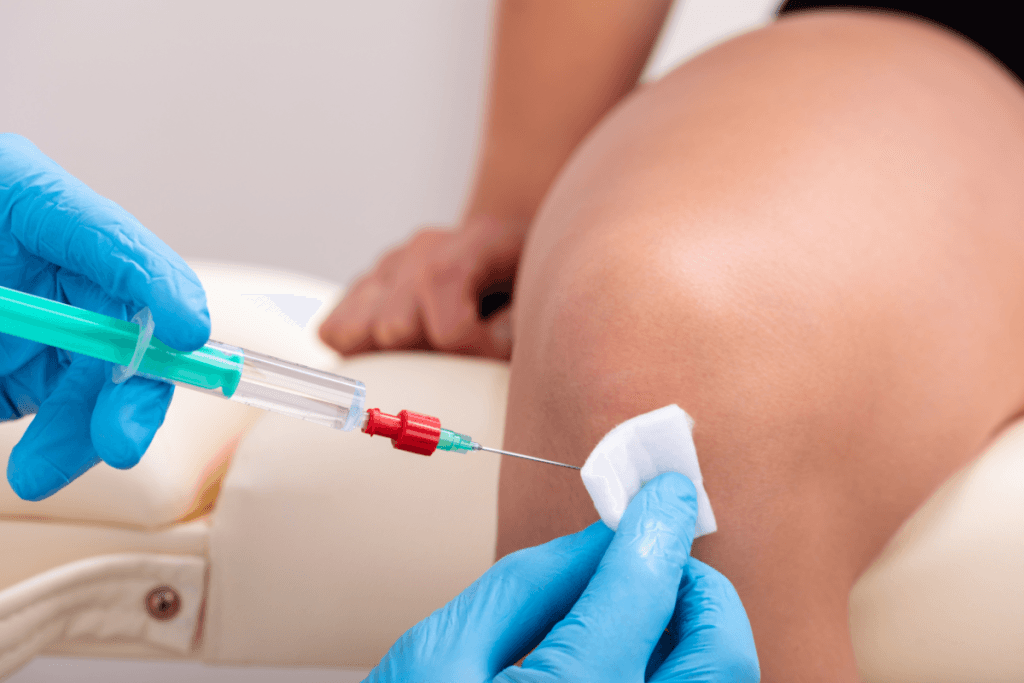
In many orthopedic and sports medicine settings, hyalgan injections are considered when knee osteoarthritis symptoms persist despite conservative care. For clinic teams, the practical questions are often less about the concept of viscosupplementation (gel-like joint lubrication) and more about fit: patient selection, risk screening, visit cadence, documentation, and product verification.
This guide focuses on operational readiness. It outlines what Hyalgan is, how hyaluronic acid products are typically positioned, and what to standardize across intake, consent, and follow-up. For broader context on comparable intra-articular options, see Types Of Gel Injections and the Orthopedic Injectables hub.
Key Takeaways
- Standardize screening, consent, and post-visit instructions
- Confirm ingredients and allergy statements from official labeling
- Plan series cadence and follow-up before scheduling starts
- Document lot numbers and injection details for traceability
- Compare HA options using workflow and risk factors
Many suppliers restrict access to verified, licensed healthcare professionals.
Clinic Workflow for hyalgan injections
From a clinic operations view, viscosupplementation sits between “procedure” and “therapy series.” That hybrid nature drives most workflow errors. Intake needs enough clinical context to justify the visit. Scheduling needs a defined series plan. Nursing support needs consistent materials, aseptic prep steps, and a standard note template. Billing and coding teams need alignment with payer policies, which can differ by plan and setting.
It helps to separate three lanes in your process. First is candidacy screening and shared decision-making documentation (why this option, why now). Second is the day-of procedure workflow (timeouts, site verification, sterility, documentation). Third is follow-up tracking (tolerance, symptom trajectory, and adverse event capture). If you want deeper detail on screening logic and contraindication handling, use Optimal Patient Selection.
Quick tip: Keep a single “viscosupplementation packet” with consent, aftercare, and a standardized procedure note.
Documentation and intake elements to standardize
Clinics tend to be most consistent when they define “minimum documentation” before the first appointment is booked. That minimum can include a diagnosis and laterality, baseline pain and function measures used by your practice, prior conservative modalities tried, and relevant comorbidities that affect procedural risk. On the day of treatment, capture injection site, approach, aseptic preparation, any aspiration performed, product identifiers, and immediate tolerance. Make room for payer-driven fields, but keep the clinical core stable across clinicians.
Use a checklist that is short enough to complete every time:
- Diagnosis and laterality documented
- Skin check and infection screen
- Allergy history reviewed and noted
- Consent and aftercare materials provided
- Product name and lot recorded
- Procedure note completed same day
- Follow-up plan and contact pathway set
When you source branded injectables through wholesale channels, expect product traceability to be supported by vetted distribution documentation.
What Hyalgan Is Made From and How It Works
Hyalgan is a hyaluronic acid (HA) product delivered by intra-articular injection. HA is a naturally occurring glycosaminoglycan (a long-chain sugar) present in synovial fluid. In osteoarthritis, synovial fluid can lose elasticity and lubricating properties, and the joint environment becomes more inflammatory.
Clinic teams often hear “gel injection” as shorthand. That phrase can confuse patients, because the product is not a permanent implant. It is better described as viscosupplementation: supplementing the joint with HA to support lubrication and shock absorption, and potentially modulate the local inflammatory environment. At a high level, the hyalgan injection mechanism of action is thought to involve restoring viscoelastic properties and interacting with synovial tissues and receptors. The exact pathways and clinical relevance vary by product and patient factors.
Ingredients and source considerations
Questions like what is hyalgan made from usually come up during allergy review and patient counseling. HA products can differ in molecular characteristics and in how the HA is sourced or processed. Some are derived from avian tissue (often described as “rooster comb” source) while others are produced by bacterial fermentation. Your clinic should rely on the current official label for hyalgan injection ingredients and any allergy statements, rather than assumptions based on older training materials. For background on avian-derived HA language patients may reference, see Understanding Rooster Comb Injections.
Why it matters: Ingredient source impacts allergy screening and informed consent wording.
In practice, many clinics position hyalgan injections as a non-surgical option for symptomatic knee osteoarthritis, alongside physical therapy, bracing, and weight-management strategies where appropriate. For a broader comparative discussion of HA options and evidence framing, see Comparing Hyalgan And Other Hyaluronic Acid Injections.
Indications, Contraindications, and Series Logistics
Hyaluronic acid injections are commonly discussed in the context of hyalgan injection knee use, especially for osteoarthritis-related pain and functional limitation. The clinical reality is that “indications” are a three-part intersection: labeled indication, patient-specific factors, and payer rules. Keep your internal protocol aligned to the official labeling and your specialty society guidance, then build intake prompts that gather the data you routinely need.
For risk screening, hyalgan injection contraindications and precautions are typically approached like any intra-articular procedure. Clinics usually screen for local skin compromise, suspected joint infection, and relevant allergy history, and they consider anticoagulation status and immunosuppression in a structured way. Policies vary by organization, so it helps to document your decision pathway and keep it consistent across providers.
Series planning and scheduling controls
From an operations standpoint, the hyalgan injection series is where variability shows up. Some HA products are administered across multiple visits, and visit intervals and total injections depend on the specific product labeling and clinical plan. Avoid hard-coding a schedule into your templates unless you tie it to the product and the ordering clinician’s plan. Instead, build a flexible “series tracker” in your EHR that captures hyalgan injection frequency, laterality, and missed-visit rules. If your practice uses multiple HA brands, keep separate series templates to reduce mix-ups across products with different hyalgan injection dosing schedule expectations.
When clinics evaluate viscosity options, they often review product families and packaging formats. For example listings, you can reference Hyalgan English 1 Syringe and alternative HA formats like Monovisc Prefilled Syringe to understand how packaging affects inventory and appointment flow.
Effectiveness and Timing: Setting Expectations
Clinicians and staff are often asked, “how long does it take for hyalgan injections to work?” The safest operational approach is to avoid overpromising timelines and to anchor discussions in variability. Symptom response can be gradual and influenced by disease severity, activity patterns, coexisting meniscal pathology, and expectations. Some practices use consistent pre- and post-series measures (pain scales, function surveys, or timed activity tests) to make follow-up more objective.
When reviewing hyalgan injection effectiveness, separate evidence sources. Clinical trials, observational studies, and patient-reported “hyalgan injection reviews” reflect different populations and biases. Online reviews can overrepresent extremes and rarely capture confounders like concurrent physical therapy, weight change, or analgesic adjustments. A practical clinic safeguard is to document what “success” means for that patient (walking tolerance, stairs, sleep disruption, analgesic reliance) before the first injection. That makes follow-up conversations clearer and reduces dissatisfaction driven by mismatched goals.
For clinics that co-manage with rehabilitation teams, it can help to align the series plan with therapy visits. See Combination Therapy Hyalgan And Physical Therapy for workflow ideas that keep documentation and scheduling coordinated.
Many practices limit distribution of branded injectables to licensed clinical channels only.
Adverse Effects, Precautions, and Post-Procedure Activity
Like any intra-articular procedure, HA injections can cause local reactions. Commonly discussed hyalgan injection side effects include transient pain at the injection site, swelling, warmth, stiffness, or a temporary increase in effusion. Serious complications are uncommon but can include infection and significant inflammatory reactions, which is why standardized aseptic technique and clear post-visit instructions matter.
Operationally, hyalgan injection risks are best managed through three levers: screening, technique consistency, and post-visit communication. Screening is where you identify red flags before the patient arrives. Technique is where you control what you can on the day. Communication is where you reduce after-hours confusion and ensure timely escalation when symptoms exceed expected post-procedure effects.
Activity guidance and “return to exercise” messaging
Patients frequently ask about exercise after hyalgan injection and about hyalgan injection recovery time. Your clinic’s answer should be consistent across staff and aligned with the treating clinician’s instructions and the product labeling. Many practices provide a brief “relative rest” message immediately after injection, then advise a gradual return to usual activities. The key is to avoid one-off advice that conflicts with what the clinician documents. Write down the standard wording you use, and include a clear threshold for when the patient should call the clinic.
Common pitfalls to address in staff training:
- Under-documenting allergy review details
- Mixing series schedules across HA brands
- Missing lot numbers in the procedure note
- Unclear after-hours escalation instructions
- Assuming “gel injection” means steroid
For a broader review of conservative pathways that clinics may coordinate alongside injections, see Exploring Non Surgical Alternatives.
How to Compare Options: HA Products, Steroids, and Alternatives
Comparisons like hyalgan vs synvisc often come up when clinics standardize formularies or respond to payer preferences. While clinicians lead clinical selection, operations teams can support a fair comparison by focusing on factors that affect workflow and risk controls. Product labels differ in ingredients, manufacturing source, packaging, and labeled schedules. Those differences can change allergy screening language, inventory turnover, and appointment cadence.
It also helps to address a recurring misconception: is hyalgan injection a steroid? It is not a corticosteroid (such as cortisone). It is an HA-based viscosupplement. When discussing hyalgan injection vs cortisone in a clinic setting, keep the comparison neutral and label-grounded. These treatments have different mechanisms, different counseling points, and different adverse event profiles. Your consent and aftercare documents should reflect that distinction clearly.
Decision factors your team can document
To keep comparisons consistent, many clinics record a short set of “decision factors” in the chart or procurement notes. This is also where confusing searches like “hyalgan supartz visco-3 dose” tend to originate, because each HA product has its own labeling. Consider documenting:
- Labeled schedule and visit burden
- Ingredient source and allergy statements
- Packaging and storage requirements
- Payer policy constraints and substitutions
For product-to-product context, you can review clinical comparisons such as Hyalgan Vs Synvisc and Hyalgan Vs Euflexxa. If your clinic uses different HA inventory, keep product references in your system precise, for example Euflexxa Prefilled Syringes as a distinct item with its own documentation fields.
Some wholesale suppliers focus on authentic, brand-name products sourced through vetted distributors.
Authoritative Sources
Use primary sources for labeling, indications, and safety language. These references can also help align your consent templates and staff scripting to the product’s official wording, which reduces inconsistency across clinicians and locations.
For neutral, authoritative reference, start with:
- DailyMed prescribing information and labeling database
- AAOS clinical practice guidelines (orthopedics)
- ACR clinical practice guidelines (rheumatology)
Further reading can be most useful when it answers a workflow question. If you are updating intake criteria or consent language, prioritize labeling and society guidance first, then use secondary analyses to refine your clinic protocol.
This content is for informational purposes only and is not a substitute for professional medical advice.