For clinics that offer viscosupplementation (joint “gel” injections), product selection affects both outcomes discussions and day-to-day operations. When your team evaluates crespine gel, you usually need answers that go beyond a brochure. You need to confirm what it contains, how it is regulated in your market, and what documentation should follow it into the patient record.
This briefing is written for licensed healthcare professionals and procurement teams. It focuses on practical verification, safety screening concepts, and how to compare hyaluronic acid (HA) options without overreaching beyond labeling.
Key Takeaways
- Confirm labeling and regulatory status before adding any HA injectable.
- Standardize documentation for lot numbers, expiry, and traceability.
- Screen for infection risk and hypersensitivity per the product IFU.
- Compare products by workflow fit, not marketing language.
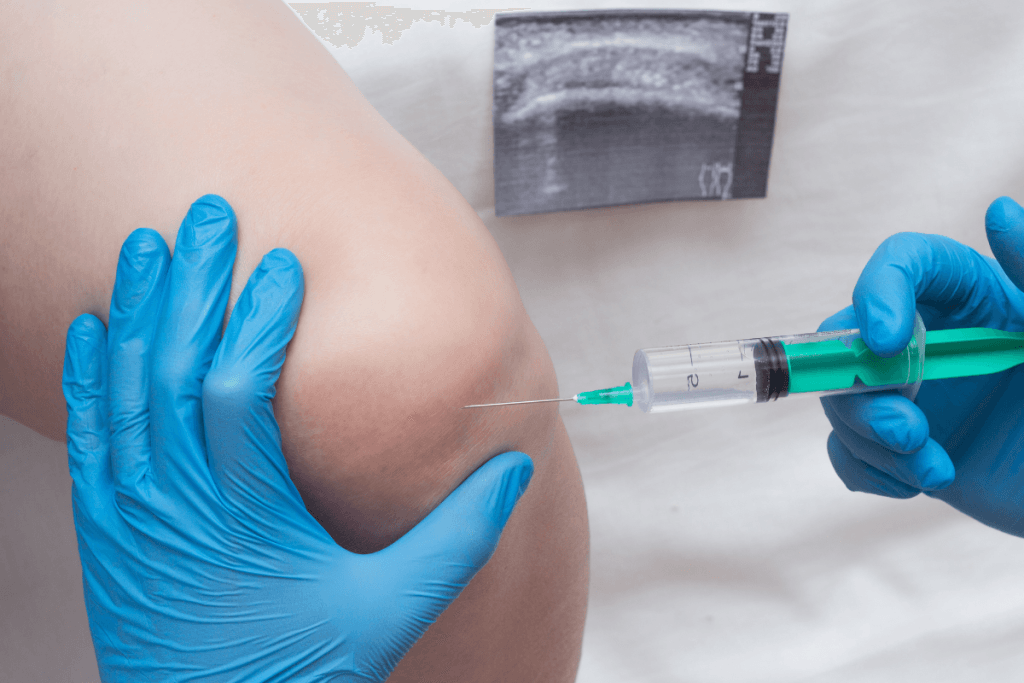
Where Viscosupplementation Fits In Knee OA Pathways
Hyaluronic acid injections are used in some settings for symptomatic knee osteoarthritis (OA). They are often discussed as an option when exercise therapy, weight management, and oral or topical analgesics are not sufficient, or when certain patients cannot tolerate other approaches. Local practice patterns vary widely, and guideline positions are not uniform across professional bodies. For a quick refresher on the category and common formats, see the site overview on Types Of Gel Injections and the background explainer Rooster Comb Injections Guide.
From an operations standpoint, the first decision is whether an HA injectable fits your service line and documentation standards. The second is whether the specific brand has consistent supply, clear instructions for use (IFU), and an appropriate regulatory footing in your jurisdiction. If your team is mapping a set of options, it can help to start with a category view such as Orthopedic Injectables and then narrow to products that match your protocols. Some clinics also keep a comparator list for patient conversations, such as Orthovisc English and other HA brands. Crespine Gel Plus may be evaluated within that same workflow, depending on local availability and status.
Access is limited to verified licensed healthcare accounts.
Mechanism And Formulation Questions To Ask
Most “gel” knee injections in this space are forms of hyaluronic acid. HA is a naturally occurring component of synovial fluid. The goal of viscosupplementation is to support joint lubrication and shock absorption, which may help some patients with symptomatic OA. Because products differ, clinics should avoid assuming that one HA syringe behaves like another in terms of viscosity, handling, or patient tolerance.
Hyaluronic Acid As A Viscosupplement
HA products can vary by molecular weight, cross-linking (a manufacturing method that can change how long a gel persists), and source material. The formulation may influence syringe feel, injection force, and how clinicians describe expectations. Many products are supplied as prefilled syringes, but excipients (inactive ingredients) may differ and can matter for patients with sensitivities. When you review a candidate product, prioritize the IFU and any official labeling first. If you are building a standardized counseling script, keep it label-aligned and focused on variability in response rather than promises of duration or magnitude of relief.
What “Plus” May Indicate In Branding
Product names that include terms like “Plus” often signal a formulation or presentation distinction within a brand family. However, the meaning is not standardized across manufacturers. Your team should confirm the stated ingredients, whether any local anesthetic is included, the intended injection schedule (single-injection versus series), and any handling constraints from the manufacturer. If you need a framework for comparing HA options with different presentations, the clinical tradeoffs discussed in Comparing Hyalgan And Other HA can help you structure your review without relying on informal comparisons.
Why it matters: Small formulation differences can change clinic workflow and patient tolerance discussions.
Crespine Gel Verification And Clinic Workflow
Before you add any intra-articular product to a knee OA pathway, align procurement, nursing, and clinical documentation. Start with identity checks: product name, manufacturer details, language on the carton and syringe, and matching lot/expiry information. For “made in which country” questions, do not rely on secondary summaries. Use primary packaging and manufacturer documentation, since contract manufacturing and regional packaging can differ by market. If your jurisdiction requires specific identifiers (for example, barcodes or device identifiers), confirm they are present and scannable before first use.
Regulatory footing can be complicated for HA injectables because classification varies by country and product. Keep your internal policy simple: document what your local regulator requires, and store supporting evidence in a way that is audit-ready. If anything is unclear (for example, “approval status” in your region), pause and verify before administration. Most clinics also benefit from a single intake template that captures product details and aligns with your adverse event reporting process.
Quick tip: Use a single lot/expiry capture step in the EHR for every injection visit.
Inventory supplied to clinics is typically obtained via vetted distribution channels.
Procurement And Documentation Checklist
- Primary label review: match name, lot, expiry.
- IFU access: store a controlled, current version.
- Traceability: record lot in the patient chart.
- Storage checks: confirm temperature and light requirements.
- Receipt log: document any damage or discrepancies.
- Staff training: align handling and aseptic setup steps.
- Waste tracking: record disposed or expired units.
Clinic workflow snapshot: verify credentials and product identity, document receipt, store per IFU, prepare aseptically, administer per clinician protocol, then record lot/expiry and any immediate reactions.
Safety Screening, Contraindications, And Follow-Up
Safety considerations for HA injections are usually discussed in three buckets: local reactions, infection risk, and hypersensitivity. Local post-injection pain, swelling, warmth, or effusion can occur with intra-articular procedures in general. Clinics should treat this as a workflow issue as much as a clinical one. Set expectations using label language, and give staff a consistent way to document symptom timing, severity, and any interventions used by the clinic. When teams search for crespine gel side effects, they are often looking for how to distinguish typical post-procedure discomfort from events that warrant escalation.
Contraindications and precautions depend on the specific product labeling. Common themes across many intra-articular products include avoiding injection into infected joints, avoiding use when there is significant skin infection at the injection site, and considering known hypersensitivity to product components. Do not generalize across brands when excipients differ. Also review your anticoagulation documentation process, your skin-prep protocol, and whether you use landmark guidance or imaging guidance, since these choices affect both risk management and charting. Recovery time language should remain conservative: many patients resume usual activities quickly, but individual reactions and clinic instructions vary.
- Common pitfalls: incomplete lot recording in EHR.
- Common pitfalls: mixing counseling language across brands.
- Common pitfalls: skipping a site skin check documentation.
- Common pitfalls: unclear plan for delayed swelling calls.
Evidence, Duration, And Interpreting Real-World Feedback
Clinicians and practice managers often ask, “How long does it last?” The most accurate answer is that duration of symptom relief varies by patient factors, OA severity, and the product used, and it should be discussed using the product’s official labeling and the broader evidence base for viscosupplementation. Avoid placing a specific timeline in templates unless it is explicitly supported by the IFU or local policy. If your team is reviewing crespine gel reviews, treat anecdotal reports as signals to investigate documentation quality and patient selection processes, not as proof of performance.
When you assess clinical studies, focus on endpoints and comparators that match your practice. Look for study design, OA grading methods, use of rescue analgesics, and how outcomes were measured (for example, pain scores or function scales). Also look for how adverse events were collected, since “minor” events can still create follow-up burden for your clinic. For structured comparisons and counseling considerations, the deeper reads Orthovisc Vs Synvisc and Synvisc In Severe OA can help you frame expectations with appropriate caution.
Comparing HA Injectables For Operational Fit
Comparisons are most useful when they are operational. Instead of asking which brand is “best,” ask which product fits your clinic’s workflow and documentation standards. For example, clinics often compare HA options by injection schedule (single vs multi-visit series), syringe presentation, and evidence quality in populations similar to their own. If you are documenting informal comparisons like “crespine gel vs synvisc,” keep the language neutral and anchored to observable differences, such as dosing schedule on the label, contraindications, and patient counseling steps.
It can also help to keep a limited set of familiar alternatives available for continuity when a product is backordered or when a clinician prefers a different presentation. Examples of comparators your team may already know include Monovisc Prefilled Syringe and Hyalgan English Syringe. For discussion on how series-based products can affect visit planning, see Tailoring Hyalgan Injection Plans. Some practices also consider adjacent regenerative services; for a separate evidence and workflow discussion, review PRP Orthopedic Advancements.
Clinics are supplied with brand-name products intended for professional use.
| Decision Factor | What To Verify | Why It Affects Workflow |
|---|---|---|
| Regulatory status | Local classification and permitted use | Impacts ordering, consent language, and audits |
| Presentation | Prefilled syringe, labeling, language | Changes prep steps and staff training |
| Visit planning | Single injection vs series on IFU | Drives scheduling, reminders, and capacity |
| Ingredients | HA plus excipients per IFU | Informs hypersensitivity screening and documentation |
| Adverse event plan | Expected reactions and escalation process | Reduces call burden and improves consistency |
Authoritative Sources
When questions arise about guideline positioning, safety language, or what “approval” means for a product class, use primary sources first. For knee OA, professional society guidance can help you structure policy discussions, even when recommendations differ. For product-specific requirements, the manufacturer’s IFU and your local regulator’s database are more reliable than summaries.
If you need to validate regulatory status or classification in your market, confirm the exact brand name, manufacturer, and any identifiers shown on packaging. Then cross-check against the applicable regulator or device/drug database. Keep a screenshot or PDF in your compliance file, and note the date accessed, since database entries can change. Also align your adverse event reporting plan with local requirements.
- AAOS knee osteoarthritis resources
- OARSI osteoarthritis guideline resources
- FDA medical device databases
Further reading and internal alignment meetings work best when your team separates clinical evidence review from procurement verification. If you decide to add crespine gel to your pathway, make the documentation steps as routine as the injection visit itself.
This content is for informational purposes only and is not a substitute for professional medical advice.